US Pharm. 2018;43(9):21-26.
ABSTRACT: Hormone replacement therapy (HRT) is the most effective treatment for the vasomotor and genitourinary symptoms associated with menopause. Adverse effects associated with HRT are dependent on various factors, including dosage, route, duration of use, timing of initiation, and choice of agent. The decision to start HRT should include a risk assessment, and HRT should be individualized to the patient. The many existing HRT options come in a wide array of dosages and dosage forms. Pharmacists should be able to counsel patients about HRT and its side effects.
The average woman will live one-third of her life in or past menopause.1 Treatment of menopausal symptoms has gained increasing importance owing to the morbidities associated with aging in women.2 Hormone replacement therapy (HRT), a term used to describe estrogen therapy, estrogen-progesterone combination therapy, or estrogen receptor agonist or antagonist therapy, continues to play a major role in the mitigation of perimenopausal and postmenopausal symptoms.3 Although each woman’s experience is different, vasomotor symptoms (VMS) and vulvovaginal symptoms are two of the most common complaints associated with the hormonal changes involved in menopause.4
Menopausal Symptoms
VMS are some of the most common and most troublesome menopausal symptoms. Hot flashes, characterized by a sudden sensation of heat in the upper chest and face that lasts 2 to 4 minutes, occur in approximately 75% of menopausal women.1 Other possible VMS are perspiration, palpitations, and anxiety.1 VMS have been associated with diminished sleep quality, irritability, difficulty concentrating, and reduced quality of life.3 Furthermore, these symptoms have been linked to cardiovascular, bone, and cognitive risks.5-9 HRT has long been recognized as the most effective treatment for VMS.4,10
In addition to bothersome VMS, many women experience vulvovaginal symptoms. It is estimated that 10% to 40% of women experience vaginal atrophy.3 Vaginal atrophy may cause vulvar pain, burning, and itching; vaginal dryness; vaginal discharge; dyspareunia (difficult or painful intercourse); and spotting or bleeding after intercourse.1 These symptoms tend to occur in the late postmenopausal stage and can lead to discomfort from tight-fitting clothing, while sitting or exercising, or with sexual activity.1 Topical HRT is typically recommended for treatment of vulvovaginal symptoms.1
In addition to alleviating the previously stated symptoms, evidence exists that standard low-dose HRT also can prevent postmenopausal hip, spinal, and nonspinal fractures.10 Benefits of HRT for VMS and genitourinary symptoms include improvements in overactive bladder, decreased sleep disturbances, reduced risk of type 2 diabetes, potential protection from coronary artery disease (CAD) and myocardial infarction, decreased risk of osteoporosis or fracture, and a potential reduction in all-cause mortality.1,10 The benefits HRT confers on bone density, however, diminish rapidly following discontinuation. Additionally, although some products have FDA approval for prevention of osteoporosis, none of them are currently approved for treatment of osteoporosis. Major risks associated with use of these agents include venous thromboembolism (VTE), ischemic stroke, increased incidence of endometrial cancer (if an unopposed estrogen is used in a woman with a uterus), and increased risk of breast cancer when HRT is used beyond 3 to 5 years.4,10
Available HRT Products
A wide array of HRT products are available in various dosage forms.4 Of the oral preparations, options include conjugated equine estrogens (CEE), micronized 17-beta estradiol (micronized to improve absorption), esterified estrogens, ethinyl estradiol (a highly potent estrogen preparation that is used at much lower dosages for HRT than for oral contraception), estrogen-progestin combination tablets, and a CEE/bazedoxifene combination tablet.4
The CEE/bazedoxifene tablet, a combination of estrogen and a selective estrogen receptor modulator, is available in the United States for menopausal VMS treatment and osteoporosis prevention. In this combination, bazedoxifene prevents estrogen-induced endometrial hyperplasia, making the administration of a progestin unnecessary. However, bazedoxifene is associated with increased risk of VTE.4 Use of bazedoxifene has been found to be safe when studied for up to 7 years in treatment of osteoporosis, but the combination has been studied only for shorter durations.11,12 VMS trials of up to 2 years’ duration have demonstrated no increase in the incidence of breast tenderness or risk of breast cancer.3 Current guidelines recommend the use of CEE/bazedoxifene in postmenopausal women with a uterus and without contraindications to treat VMS and help prevent bone loss, but do not specify duration of therapy.1 Based on available evidence, treatment of VMS with CEE/bazedoxifene for up to 2 years appears safe; safety of use beyond 2 years remains unclear.
HRT is also available in various topical applications. Transdermal patch preparations of 17-beta estradiol or estrogen-progestin combinations are available. Other topical preparations include gel packets or metered-dose pumps, sprays, and emulsions.4 Depot injections may also be considered, but these products are rarely used in the setting of HRT.4 A summary of available systemic HRT formulations is given in TABLE 1.13-15
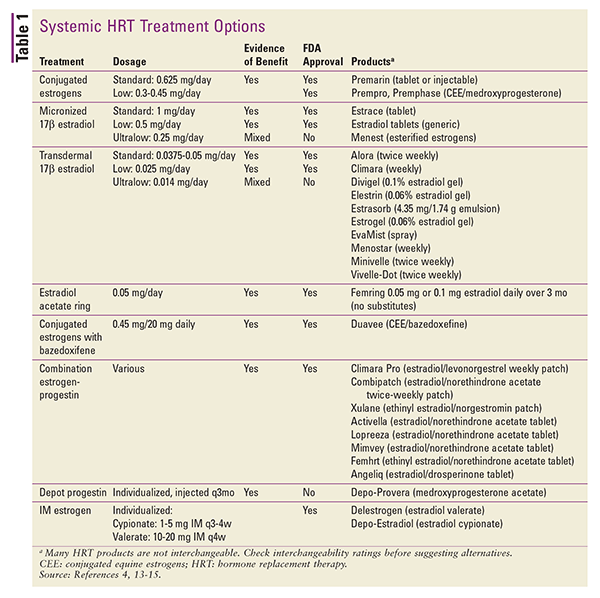
In converting from one dosage form to another, differences in route of administration and product potency can be accounted for by using estimated dose equivalents.4 Dose equivalents for oral preparations are as follows: 1 mg micronized 17-beta estradiol equals 1.25 mg piperazine estrone sulfate. In conversions between oral and transdermal estrogen preparations, 0.05 mg/24 hours transdermal is equal to approximately 0.625 mg daily by mouth.4
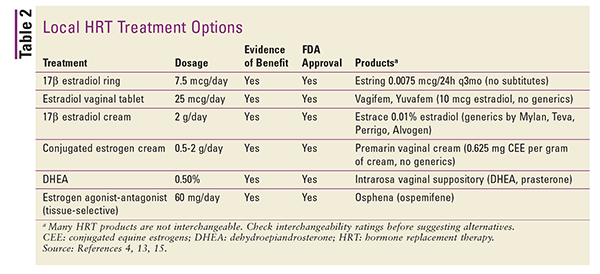
Local preparations are generally low-dose and are used specifically for the management of symptoms of vulvovaginal atrophy.4 Higher doses may be used for treatment of systemic VMS, but this is generally not recommended.4 Local preparations include vaginal rings, tablets, and creams. Refer to TABLE 2 for a summary of available local HRT formulations.4,13,15
Current Guideline Recommendations
Three organizations—the American College of Obstetricians and Gynecologists, the North American Menopause Society (NAMS), and the Endocrine Society—have produced guidelines on the use of HRT in menopause. As noted in these guidelines, indications for HRT include management of VMS associated with menopause and treatment of vulvovaginal effects in the absence of bothersome systemic symptoms.1,3,4 Estrogen alone or in combination with a progestin was found to reduce weekly VMS by 75% and significantly reduce symptom severity compared with placebo.4
In women aged younger than 60 years who are less than 10 years post menopause, have bothersome VMS, and have no contraindications to HRT or excess cardiovascular or breast cancer risk, use of estrogen-only therapy is recommended for those without a uterus. Addition of a progestin is recommended in patients with a uterus to prevent endometrial hyperplasia and potential endometrial cancer.1,3,4 Currently, the required progestin exposure is unclear, but many providers give progestins in a 14-day cycle each month.4 For women aged older than 60 years or who are more than 10 years post menopause, consideration of other (nonhormonal) therapies is recommended instead of HRT.3
In women with an elevated risk of cardiovascular disease (CVD; defined as >10% 10-year risk of CVD per the American College of Cardiology/American Heart Association cardiovascular risk estimator tool), nonhormonal therapies are preferred.3 In women with moderate CVD risk, transdermal estrogen with or without progestin is preferred over oral therapy, as these preparations have less effect on blood pressure, lipids, and carbohydrate metabolism.1 In patients with an elevated risk of VTE, use of nonoral routes at the lowest effective dose is preferred. Lastly, in patients with high or intermediate breast cancer risk, preference is given to nonhormonal therapies.4
If HRT is selected, all guidelines recommend use of the lowest effective dose.1,4,10 One benefit of lower doses of HRT is the better side-effect profile compared with high-dose regimens.4 There is no currently preferred route of therapy for treatment of VMS; therefore, route of administration should be guided by patient preference. It should be noted that transdermal products may be associated with a lower incidence of adverse effects, such as VTE, but studies are still being conducted.10
For the treatment of genitourinary symptoms in women without a history of hormone-dependent cancers, low-dose vaginal estrogen may be used.1 For vaginal symptoms only, guidelines recommend against the addition of progestin regardless of whether the uterus is present. This therapy may also be appropriate in patients with a history of hormone-dependent cancers, but in those cases, the choice of therapy should be based on a shared decision-making process that includes discussion of the risks and benefits. Potential benefits of vaginal estrogen include rapid improvement in vaginal and urinary symptoms and possible prevention of recurrent urinary tract infections. Nonestrogen FDA-approved and guideline-concordant therapies used to relieve dyspareunia include ospemifene and intravaginal dehydropiandrosterone.16,17
Progestin-only therapy, testosterone, plant-based alternative nonhormonal therapies, and compounded bioidentical hormones lack data and generally are not recommended.1,4 The NAMS states that prescribers may consider compounded HRT in situations where a patient is unable to tolerate FDA-approved therapies.3 Nonhormonal treatment options for vasomotor symptoms include selective serotonin reuptake inhibitors and selective serotonin-norepinephrine reuptake inhibitors, clonidine, and gabapentin, and nonhormonal treatment for vaginal symptoms include lubricants, estrogen agonist-antagonists, and herbal remedies.4 Evidence supporting the use of nonhormonal therapies varies, and not all options have proven evidence or an FDA-approved indication.4
Monitoring of HRT should include regular breast cancer screenings during and following completion of therapy. Recurrent unscheduled bleeding should be assessed quickly.1 Duration of therapy should be revisited at least annually, and the shortest treatment duration is preferred.1 When patients and providers decide to discontinue HRT, either a taper or an abrupt discontinuation strategy may be used. Evidence is lacking regarding methods of discontinuation; therefore, shared decision making is recommended for the process of discontinuation.1,4
Drug-Drug Interactions
The majority of commercially available HRT products (TABLE 1) undergo liver metabolism mediated by the CYP450 system.15 Specifically, estrogen products are partially metabolized by CYP3A4; therefore, strong inducers of CYP3A4 can reduce the efficacy of estrogen HRT and strong inhibitors can increase serum concentrations of estrogen. Additionally, other drug substrates for these enzymes can interfere with the metabolism of HRT.15
Severe and Common Side Effects
Major side effects associated with HRT include CVD, breast cancer, endometrial or ovarian cancer, stroke, and VTE.1 Unopposed estrogen use increases the risk of endometrial hyperplasia and cancer, hence the recommendation for addition of progestin in women with a uterus. Regarding the risk of breast cancer, there appears to be a small risk of developing breast cancer when a woman is on estrogen for 5 years.3 The current literature is inconsistent regarding determining the risk of developing ovarian cancer from HRT.3 More randomized, controlled trials are needed to ascertain the true risk of developing ovarian cancer with HRT use.1 The effects of HRT on coronary artery disease (CAD), stroke, and VTE vary depending on a woman’s age at time of initiation or time since onset of menopause. It appears that women taking HRT for more than 10 years from onset of menopause are at increased risk for developing CAD.3 Overall, studies suggest that lower-dose oral and transdermal formulations confer a decreased risk of stroke and VTE compared with standard-dose HRT.3 Patients who are older than 60 years when initiated on HRT or who are more than 10 years from the start of menopause are at increased risk for these adverse effects. Furthermore, women taking HRT with estrogen either alone or in combination are at increased risk for gallstones, cholecystitis, and cholecystectomy.3 The risk of gallbladder disease has been associated with use of oral HRT and may not exist with topical or transdermal formulations.1,3
Contraindications
Professional society guidelines have not specified a list of absolute or relative contraindications to HRT.2 Therefore, as with most clinical decision making, an individualized risk-benefit assessment is important. In general, HRT should be avoided in patients with unexplained vaginal bleeding; history of stroke or transient ischemic attack; myocardial infarction; pulmonary embolism or VTE; breast or endometrial cancer; active liver disease; known protein C, protein S, or antithrombin deficiency or other thrombophilic disorders; and known or suspected pregnancy.1 Additionally, caution should be exercised in patients with diabetes, gallbladder disease, hypertriglyceridemia (>400 mg/day), hypoparathyroidism, benign meningioma, intermediate or high risk for breast cancer, high risk for heart disease, or migraine with aura.2
Counseling Points
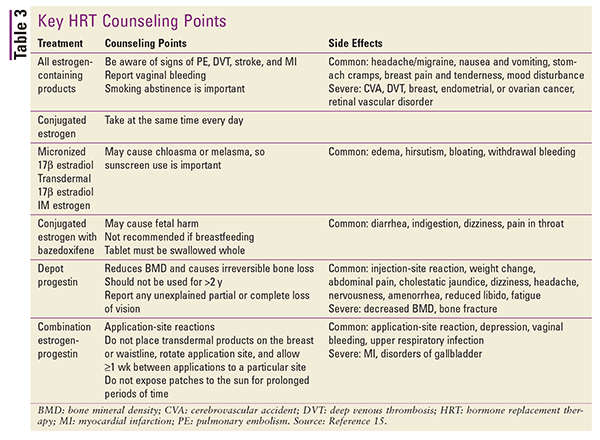
Refer to TABLE 3 for a summary of key patient counseling points and side effects for the various types of HRT.15
Conclusion
HRT, which is available in a wide array of preparations, is the most effective treatment for both VMS and genitourinary symptoms. Because the risks associated with HRT are different for each woman, treatment should be individualized and aimed at maximizing efficacy, minimizing therapy duration, and reducing the risk of adverse effects. In scenarios in which HRT is not an option, nonhormonal treatment options for both VMS and genitourinary symptoms are available, but efficacy and FDA approval of these products vary; therefore, efficacy data for these products should be reviewed.
REFERENCES
1. Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015;100(11):3975-4011.
2. Shifren JL, Schiff I. Role of hormone therapy in the management of menopause. Obstet Gynecol. 2010;115(4):839-855.
3. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of the North American Menopause Society. Menopause. 2017;24(7):728-753.
4. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123(1):202-216.
5. Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, et al. Hot flashes and subclinical cardiovascular disease: findings from the Study of Women’s Health Across the Nation Heart Study. Circulation. 2008;118(12):1234-1240.
6. Thurston RC, Kuller LH, Edmundowicz D, Matthews KA. History of hot flashes and aortic calcification among postmenopausal women. Menopause. 2010;17(2):256-261.
7. Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, et al. Hot flashes and carotid intima media thickness among midlife women. Menopause. 2011;18(4):352-358.
8. Crandall CJ, Aragaki A, Cauley JA, et al. Associations of menopausal vasomotor symptoms with fracture incidence. J Clin Endocrinol Metab. 2015;100(2):524-534.
9. Maki PM. Verbal memory and menopause. Maturitas. 2015;82(3):288-290.
10. North American Menopause Society. The 2012 hormone therapy position statement of: The North American Menopause Society. Menopause. 2012;19(3):257-271.
11. Palacios S, Silverman SL, de Villiers TJ, et al. A 7-year randomized, placebo-controlled trial assessing the long-term efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: effects on bone density and fracture. Menopause. 2015;22(8):806-813.
12. Archer DF, Freeman EW, Komm BS, et al. Pooled analysis of the effects of conjugated estrogens/bazedoxifene on vasomotor symptoms in the selective estrogens, menopause, and response to therapy trials. J Womens Health (Larchmt). 2016;25(11):1102-1111.
13. FDA. Orange book: approved drug products with therapeutic equivalence evaluations. Product details for ANDA 204726. www.accessdata.fda.gov/scripts/cder/ob/results_product.cfm?Appl_Type=A&Appl_No=204726. Accessed March 18, 2018.
14. FDA. Drugs@FDA: FDA approved drug products. www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Accessed June 17, 2018.
15. DrugDex [Micromedex Solutions; subscription database]. Greenwood Village, CO: Truven Health Analytics, Inc. www.micromedexsolutions.com. Accessed June 15, 2018.
16. Constantine G, Graham S, Portman DJ, et al. Female sexual function improved with ospemifene in postmenopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric. 2015;18(2):226-232.
17. Labrie F, Archer DF, Koltun W, et al. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23(3):243-256.
To comment on this article, contact rdavidson@uspharmacist.com.





