US Pharm. 2010:45(1):15-21.
Every year, approximately 13,000 women are diagnosed with cervical cancer, leading to over 4,000 deaths. In 2016, there were 12,984 new cases of cervical cancer reported, with 4,188 women dying due to cervical cancer in the United States. These numbers translate to eight in every 100,000 women developing cervical cancer, and two in every 100,000 dying because of this particular cancer. Despite being a serious and life-threatening condition, the 5-year relative survival rate for cervical cancer remains high at 67.6%. Over the past 40 years, the number of new cases and deaths have significantly diminished because of increased vaccinations and screenings. Almost all cases of cervical cancer are caused by specific strains of human papillomavirus (HPV).1
HPV is the most common sexually transmitted infection, affecting approximately 79 million Americans and causing 14 million new infections annually, predominantly in persons aged 15 to 24 years.2 Most sexually active individuals will be infected with HPV at some point during their lifetime but may never know it; most infections are asymptomatic and usually resolve spontaneously within 2 years.2-4 However, there are cases in which HPV remains active and can lead to warts, precancerous or dysplastic lesions, and several types of cancers.3
Although there is no cure for HPV infection, there are treatment options available for the various clinical manifestations of the infection. In addition, routine screenings for and early detection of precancerous changes through the use of Papanicolaou (Pap) testing have greatly reduced cases of, and deaths from, cervical cancer.2,3 Most recently, the introduction of vaccines against HPV has greatly decreased HPV prevalence among young females.5 The availability of a vaccine provides pharmacists with the opportunity to become more active in HPV education and prevention.
Human Papillomavirus
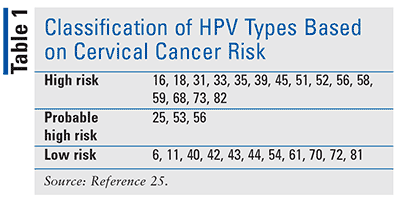
HPV is a small, double-stranded DNA virus. Of the more than 100 HPV types identified, more than 40 of these are capable of infecting mucosal epithelium. They are classified according to their risk of causing cervical cancer (TABLE 1). Infection with low-risk HPV types is usually associated with benign or low-grade cervical-cell abnormalities, laryngeal papillomas, and cutaneous or genital warts, with almost 90% of all anogenital warts caused by HPV 6 and 11. Infection with high-risk types can cause both low-grade and high-grade cervical-cell abnormalities, which are considered precursors to cancers. High-risk HPV is detected in approximately 99% of all cervical cancers. Epidemiological data collected from case-control and prospective cohort case studies have specifically described HPV subtypes 16 and 18 as the most common strains to be associated with cervical cancer. High-risk HPV types are also responsible for 90% of anal cancers, 75% of vaginal cancers, 70% of oropharyngeal cancers, and 63% of penile cancers.2,6-8
Risk Factors
HPV is transmitted through contact with infected genital skin or mucosa, usually by sexual activity. Transmission is often between asymptomatic persons. Risk factors are primarily related to sexual behavior. Genital contact is the leading risk factor, and risk increases with the number of sexual partners. Other risk factors include young age of first sexual experience, history of sexually transmitted diseases, and failure to use condoms consistently.2,9-11
Cervical Cancer Screening
Screening for HPV and cervical cancer is important for early detection and treatment. Cervical cancer screening does not prevent HPV infection, but it can detect precancerous changes, helping to prevent most cervical cancers and deaths. Screening methods include cervical cytology and HPV DNA testing. Cytology testing (conventional or liquid-based), otherwise known as the Pap test, is the preferred method for screening due to its ability to detect cytological precancerous changes. HPV DNA testing detects any of the high-risk types of HPV that are most commonly found to cause cervical cancer.
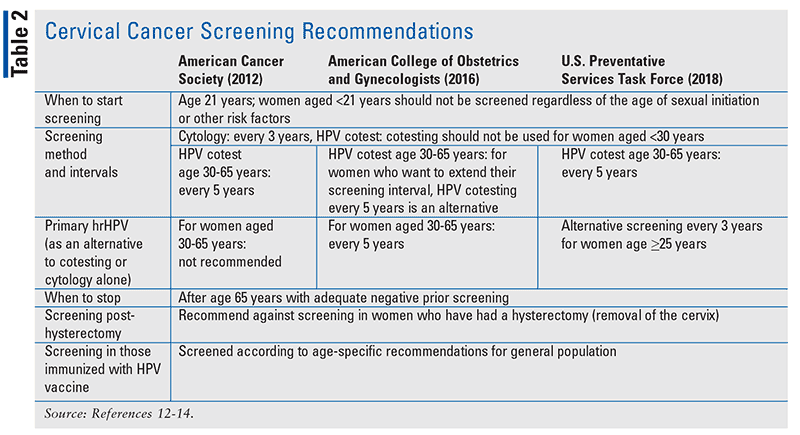
Various professional groups such as the U.S. Preventive Services Task Force, the American Cancer Society, and the American College of Obstetricians and Gynecologists have published cervical cancer screening recommendations (TABLE 2). For most women aged 21 years and older, a Pap test is recommended every 3 years; cervical cancer is rare prior to this age, and screening earlier than age 21 years would lead to more harm than benefit. As of 2018, the HPV DNA test is now an option for primary cervical cancer screening, but the organizations have differing recommendations. No matter the screening method, it is important to stress the message of regular screening. Once a woman is older than age 65 years, screenings are generally no longer recommended as long as there has been adequate negative prior screening results and no prior diagnosis of cervical intraepithelial neoplasia grade 2 or higher.12-14
Prevention: the HPV Vaccine
Since June 2006 there has been a vaccine available to help prevent new HPV infections and HPV-associated disease, including some cancers in young women and men. Currently there is one licensed vaccine available in the U.S., Gardasil 9, a nine-valent HPV (9vHPV) vaccine. It is approved for use in girls/women and boys/men aged 9 to 45 years. The 9vHPV vaccine provides protection against 9 different HPV types: 6, 11, 16, 18, 31, 33, 45, 52, and 58. Epidemiological studies have suggested that the 9vHPV vaccine could prevent an estimated 90% of cervical, vulvar, and vaginal cancers and up to 85% of high-grade cervical disease as well as preventing up to 90% of HPV-related anal cancers and genital warts in men and women worldwide. Data from a randomized, double-blind efficacy, immunogenicity, and safety study of the 9vHPV vaccine demonstrated that the 9vHPV vaccine was highly efficacious in preventing HPV infection, abnormal cervical cytology, histopathologically confirmed high-grade anogenital tract disease, and cervical procedures associated with the HPV types included in the HPV vaccine. Vaccine efficacy was sustained for up to 6 years, and more than 99% of patients seroconverted to all nine HPV vaccine types.15,16
Vaccine Safety
A study reviewed the national Vaccine Adverse Event Reporting System from 2014 to 2017 to determine the frequency of serious events reported following 9vHPV vaccine administration. There were 7,244 reports identified, with more than 97% of them considered as nonserious; during this time approximately 28 million vaccine doses were distributed. The most frequently reported adverse events included syncope, headache, dizziness, and soreness or redness at the injection site. Another study evaluated data files collected at six Vaccine Datalink sites for the first 2 years after the licensure of the 9vHPV vaccine. During this time approximately 840,000 doses were administered to patients aged 9 to 26 years, and no new safety concerns were identified.17,18
Dosing Recommendations
The Advisory Committee on Immunization Practices currently recommends that the 9vHPV vaccine be administered routinely to all children aged 11 to 12 years; vaccination can begin as early as age 9 years. In those who are not adequately vaccinated, catch-up HPV vaccination is recommended for all individuals up to age 26 years. Adults who are aged 27 through 45 years and are not adequately vaccinated against HPV may receive the vaccine based on a shared clinical decision with a healthcare provider; the public health benefit of HPV vaccination in this age range is minimal.
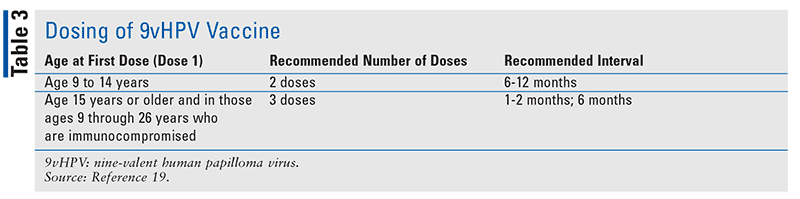
The number of doses needed will vary according to the age at which the initial dose is administered (TABLE 3). Children who receive any dose prior to their 15th birthday will need only two doses to complete the series compared with those who started vaccinating after their 15th birthday; they will require completion of a three-dose series. Two doses of the 9vHPV vaccine administered to children aged 9 to 14 years, separated by 6 or 12 months, demonstrated noninferiority to responses in young girls and women aged 16 to 26 years completing a three-dose series. In addition, geometric mean titers were significantly higher for all 9vHPV types in those who received two doses between ages 9 and 14 years compared with those who were aged 16 to 26 years and received three doses. Allowing for a two-dose series in children will potentially improve adherence to HPV vaccination programs.19-21
Role of the Pharmacist
Pharmacists can have a tremendous impact on HPV education and immunization rates. Currently, only about half of all adolescents are considered up to date with their HPV vaccine, and 66% of adolescents aged 13 to 17 years received their first dose to start the series. These rates are still below the goal of 80% set by Healthy People 2020. Because pharmacists are readily recognized as a provider of immunizations by patients, physicians, and many national organizations, including the CDC, they should be at the forefront providing both education and immunizations for this important vaccine. Even if a pharmacist is not allowed to provide the HPV vaccine, he or she should still be able to answer questions from both parents and patients about the vaccines, helping to dispel myths and address concerns regarding HPV and its prevention.22,23
Conclusion
Human papillomavirus continues to be the most commonly transmitted sexual disease. Despite the availability of a vaccine to prevent its spread, approximately 14 million new cases are diagnosed each year. Although this infection is usually self-limiting, persistent infection with high-risk HPV types can ultimately lead to cancer; HPV is responsible for nearly all cases of cervical cancer. The HPV vaccine represents an important advance in HPV prevention. An analysis demonstrated that after 5 to 8 years of vaccination, the prevalence of HPV 16 and 18 significantly decreased by 83% in girls between the ages of 13 and 19 years, by 66% in women aged 20 to 24 years, and by 37% in those aged 25 to 29 years.24 Despite its availability and known benefits, immunization rates remain inadequate. Pharmacists need to take a more active role in providing education to parents and patients about this very important vaccine.
Patient Information
How can I prevent infection with HPV?
Because HPV is a sexually transmitted disease, the best and most reliable way to prevent infection is through sexual abstinence. Condom use can also lower the risk of being exposed to HPV, but it does not fully protect against acquiring the infection since HPV can be present in areas outside of the coverage of the condom. Vaccination with the HPV vaccine, especially at an early age prior to any exposure to the virus, can prevent against infection and cancers. Implementation of the vaccine has led to a significant drop in the prevalence of HPV and HPV-related cancers.
Why should I get the vaccine?
Almost 79 million Americans are currently infected with HPV. Health problems related to HPV include genital warts and cervical and other types of cancer. The vaccine can help prevent HPV infections and related consequences. It can prevent more than 90% of these cancers.
Who should get vaccinated?
The HPV vaccine is recommended for everyone through age 26 years. Vaccination usually begins at age 11 or 12 years but can be given as early as age 9 years. If you are older than age 26 years, the decision to vaccinate should be individualized and discussed with a healthcare provider.
How many times do I have to receive the vaccine?
The number of doses will depend on when you receive your first dose. If the first dose is given when you are age 9 to 14 years, you will only need two doses. If you started the vaccine after your 15th birthday or you have an immunocompromising condition, you will need three doses.
Is the vaccine safe?
The most common side effects are mild. They include pain, redness, and swelling at the injection site. You may also experience dizziness, fainting, nausea, and headache. Fainting is more likely to occur in adolescents. These are all side effects that are seen after the administration of any vaccine.
Does this vaccine protect against all types of HPV?
There are over 100 types of HPV. This vaccine provides protection against nine of the most common types of HPV that cause cancers as well as those that cause genital warts.
Why do I have to give my child this vaccine now?
Vaccination is the best way to protect your child from future HPV infections. The vaccine is more effective when given before a person becomes sexually active.
Do I still have to go for my cervical cancer screenings if I received the vaccine?
Yes, even though you have been vaccinated, routine cervical cancer screening is still needed. Although the vaccine protects against the nine most common HPV types, you may still be at risk for some cancers from HPV.
REFERENCES
1. U.S. Cancer Statistics Data Visualizations. https://gis.cdc.gov/grasp/USCS/DataViz.html. Accessed December 18, 2019.
2. CDC. Pinkbook. Epidemiology and prevention of vaccine preventable diseases. Human papillomavirus. Published September 25, 2019. www.cdc.gov/vaccines/pubs/pinkbook/hpv.html. Accessed December 15, 2019.
3. CDC. Manual for the Surveillance of Vaccine Preventable Diseases. Chapter 5: Human papillomavirus. Published March 29, 2019. www.cdc.gov/vaccines/pubs/surv-manual/chpt05-hpv.html. Accessed December 15, 2019.
4. Moscicki AB, Shiboski S, Broering J, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr. 1998;132(2):277-284.
5. Markowitz LE, Liu G, Hariri S, et al. Prevalence of HPV after introduction of the vaccination program in the United States. Pediatrics. 2016;137(3):e20151968.
6. Saraiya M, Unger ER, Thompson TD, et al. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst. 2015;107(6):djv086.
7. CDC. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. August 29, 2014. www.cdc.gov/mmwr/preview/mmwrhtml/rr6305a1.htm. Accessed December 15, 2019.
8. Cogliano V, Baan R, Straif K, et al. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6(4):204.
9. Dempsey AF. Human papillomavirus: the usefulness of risk factors in determining who should get vaccinated. Rev Obstet Gynecol. 2008;1(3):122-128.
10. Juckett G, Hartman-Adams H. Human papillomavirus: clinical manifestations and prevention. Am Fam Physician. 2010;82(10):1209-1213.
11. Chelimo C, Wouldes TA, Cameron LD, Elwood JM. Risk factors for and prevention of human papillomaviruses (HPV), genital warts and cervical cancer. J Infect. 2013;66(3):207-217.
12. Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320(7):674-686.
13. Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147-172.
14. Committee on Practice Bulletins—Gynecology. Practice Bulletin No. 168: Cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111-e130.
15. Denny L. Nine-valent human papillomavirus vaccine: great science, but will it save lives? Lancet. 2017;390(10108):2123-2124.
16. Huh WK, Joura EA, Giuliano AR, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16-26 years: a randomised, double-blind trial. Lancet. 2017;390(10108):2143-2159.
17. Shimabukuro TT, Su JR, Marquez PL, et al. Safety of the 9-valent human papillomavirus vaccine. Pediatrics. 2019;144(6).
18. Donahue JG, Kieke BA, Lewis EM, et al. Near real-time surveillance to assess the safety of the 9-valent human papillomavirus vaccine. Pediatrics. 2019;144(6).
19. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65(49):1405-1408.
20. Meites E, Szilagyi PG, Chesson HW et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68(32):698-702.
21. Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316(22):2411-2421.
22. CDC. Understanding HPV coverage. Published October 18, 2018. www.cdc.gov/hpv/partners/outreach-hcp/hpv-coverage.html. Accessed December 17, 2019.
23. Healthy People 2020. Immunization and infectious diseases. www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed December 17, 2019.
24. Drolet M, Bénard É, Pérez N, et al. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. The Lancet. 2019;394(10197):497-509.
25. Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518-527.
To comment on this article, contact rdavidson@uspharmacist.com.