US Pharm. 2020;45(7/8):60-CV3.
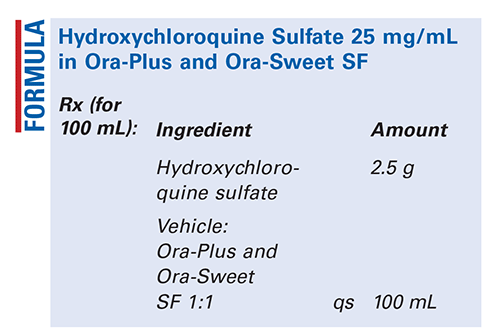
Method of Preparation: Calculate the required quantity of each ingredient for the total amount to be prepared. Accurately weigh or measure each ingredient. Place the hydroxychloroquine sulfate tablets or powder in a suitable container. Prepare vehicle by combining Ora-Plus 50 mL with Ora-Sweet SF 50 mL and mixing well. If using tablets, add a small amount of vehicle to cover the tablets and allow the mixture to soak for 15 minutes. Mix well to form a smooth paste. If using powder, add a small amount of vehicle and mix to form a smooth paste. Add a sufficient amount of vehicle to make the contents pourable. Transfer the contents stepwise and quantitatively to a calibrated container, using the remainder of vehicle. Add sufficient vehicle to bring to final volume, and mix well. Package and label.
Use: Hydroxychloroquine sulfate oral suspension has been used in the treatment of COVID-19 viral infections in patients who cannot swallow the oral tablets.
Packaging: Package suspension in tight, light-resistant containers.
Labeling: Keep out of reach of children. Discard after ____ [time period]. Shake well.
Stability: A beyond-use date of not more than 90 days may be used for this preparation when stored at refrigerated or controlled room temperature.1
Quality Control: Quality-control assessment can include weight/volume, pH (powder, 3.6-4.6; tablets, 4.4-5.4), specific gravity, active drug assay, color, rheologic properties/pourability, physical observation, and physical stability (discoloration, foreign materials, gas formation, mold growth).1-4
Discussion: COVID-19, which is caused by a coronavirus called SARS-CoV-2, is especially serious for older adults and anyone with severe underlying medical conditions, such as heart or lung disease or diabetes. Pharmacist involvement in the pandemic includes compounding oral liquids for children and the elderly, as well as many other activities related to patient care.
Although no approved coronavirus treatments exist at this time, a number of drugs are being widely used, including remdesivir, hydroxychloroquine sulfate, chloroquine sulfate, azithromycin, convalescent plasma, and others. Hydroxychloroquine sulfate has been around for decades and is routinely used to treat certain types of malaria, lupus erythematosus, and rheumatoid arthritis. This hydroxychloroquine sulfate compounded oral suspension has been proposed and is now being considered as an official compounding monograph in the USP.
Hydroxychloroquine sulfate (Plaquenil, C18H26ClN3O.H2SO4, MW 433.95) occurs as a white or practically white, odorless, crystalline powder with a bitter taste. Its solutions have a pH of about 4.5. Hydroxychloroquine sulfate exists in two forms, one melting at approximately 240°C and the other at about 198°C. Hydroxychloroquine sulfate is freely soluble in water and practically insoluble in alcohol. Plaquenil 200-mg tablets contain the equivalent of 155 mg of the base for oral administration; inactive ingredients include dibasic calcium phosphate, hydroxypropyl methylcellulose, magnesium stearate, polyethylene glycol 400, polysorbate 80, starch, and titanium dioxide.1,5
Ora-Plus is an oral suspending vehicle that accepts dilution of up to 50% or more with water, flavoring agents, or syrups while retaining its suspending properties. It has a pH of approximately 4.2 and an osmolality of about 230 mOsm/kg. Ora-Plus is a thixotropic vehicle with a viscosity of approximately 1,000 cps at 25°C. It contains purified water, microcrystalline cellulose, sodium carboxymethylcellulose, xanthan gum, carrageenan, sodium phosphate, and citric acid as buffering agents; simethicone as an antifoaming agent; and potassium sorbate and methylparaben as preservatives.6
Ora-Sweet SF sugar-free syrup is a flavoring vehicle for oral extemporaneous preparations. It is a sugar-free and alcohol-free syrup flavored with a citrus-berry flavor blend. Ora-Sweet SF is buffered to a pH of approximately 4.2, and it may be used alone or in combination with other vehicles. Ora-Sweet SF will tolerate a dilution to 50% with dissolved actives in water or suspending agents while still retaining an acceptable taste. It has an osmolality of 2,150 mOsm/kg. Ora-Sweet SF contains water, sodium saccharin, xanthan gum, glycerin, sorbitol, citric acid, and sodium citrate as buffers; methylparaben, propylparaben, and potassium sorbate as preservatives; and flavoring agents.7
REFERENCES
1. U.S. Pharmacopeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; June 2020.
2. Allen LV Jr. Standard operating procedure for quality assessment of oral and topical liquids. IJPC. 1999;3:146-147.
3. Allen LV Jr. Summary of quality-control testing for sterile and nonsterile compounded preparations, part 1: physical and chemical testing. IJPC. 2019;23(3):211-216.
4. Allen LV Jr. Summary of quality-control testing for sterile and nonsterile compounded preparations, part 2: microbiological testing. IJPC. 2019;23(4):299-303.
5. RxList. Plaquenil. www.rxlist.com/plaquenil-drug.htm. Accessed June 4, 2020.
6. Ora-Plus product information. Allegan, MI: Perrigo; 2018.
7. Ora-Sweet SF product information. Allegan, MI: Perrigo; 2018.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





