US Pharm. 2021;46(8):58-59.
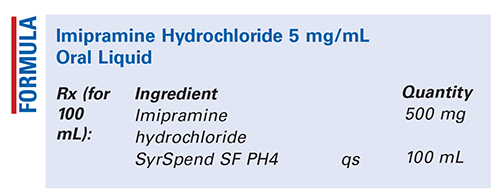
Method of Preparation: Calculate the required quantity of each ingredient for the total amount to be prepared. Accurately weigh or measure each ingredient. If tablets are used, reduce the particle size of the imipramine hydrochloride tablets to a fine powder; otherwise, place the imipramine hydro-chloride bulk powder in a mortar. Add a small portion of the SyrSpend SF PH4 to the powder; mix to form a smooth paste. Geometrically, add sufficient SyrSpend SF PH4 to final volume, mixing after each addition. Package and label.
Use: Imipramine hydrochloride has been used in the treatment of enuresis (i.e., bedwetting) in pediatric patients.
Packaging: Package in tight, light-resistant containers.1
Labeling: Keep out of reach of children. Use only as directed. Shake well. Discard after ____ [time period].
Stability: A beyond-use date of up to 90 days, when packaged in low-actinic, light-resistant prescription containers and stored at either room or refrigerated temperatures, may be used for this preparation. After 90 days, 97.59% of the drug was found to remain at refrigerated temperature, and 99.07% remained at room temperature.1,2
Quality Control: Quality-control assessment can include weight or volume, pH, specific gravity, active drug assay, color, rheologic properties/pourability, physical observation, and physical stability (discoloration, foreign materials, gas formation, and mold growth).3,4
Discussion: Nocturnal enuresis is a common problem, affecting an estimated 5 million to 7 million children in the United States and occurring more often in boys than in girls. The evaluation of nocturnal enuresis requires a thorough history, a complete physical examination, and urinalysis. Treatment options include nonpharmacologic and pharmacologic measures. Continence training should be incorporated into the treatment regimen. The use of a bed-wetting alarm has a high cure rate and the lowest relapse rate; however, some families may have difficulty with this treatment approach. Desmopressin and imipramine are the primary medications used to treat nocturnal enuresis.
Imipramine hydrochloride (Tofranil, C19H24N2.HCl, MW 316.87) occurs as a white to off-white, odorless or practically odorless, crystalline powder that is freely soluble in water and in alcohol. Tofranil (imipramine hydrochloride USP), the original tricyclic antidepressant, is a member of the dibenzazepine group of compounds. Tofranil is supplied in tablet form for oral administration with the following inactive ingredients: calcium phosphate, cellulose compounds, docusate sodium, iron oxides, magnesium stearate, polyethylene glycol, povidone, sodium starch glycolate, sucrose, talc, and titanium dioxide.5
SyrSpend SF PH4 is available as a liquid and as a dry powder for reconstitution. The liquid is preserved with less than 0.1% sodium benzoate, and it is buffered to a pH of 4.2. SyrSpend SF PH4 liquid is available either as an unflavored or a cherry-flavored vehicle. The powder for reconstitution is preservative free, is buffered to a pH of 4.2, and is available as a preweighed powder to make 100 mL (containing 6.5 g powder) or 200 mL (containing 13.0 g powder). The SyrSpend SF PH4 powder for reconstitution is unflavored.6
The SyrSpend products include SyrSpend SF Alka Cherry (Preservative Free); SyrSpend SF Alka Unflavored (Preservative Free); SyrSpend SF Cherry; SyrSpend SF Grape; SyrSpend SF PH4 Dry (Preservative Free); SyrSpend SF Powder Dry (Preservative Free); and SyrSpend SF Unflavored. SyrSpend products are oral starch-based suspension vehicles containing a patented Active Suspending Technology that holds active pharmaceutical ingredients’ particles in suspension and accelerates redistribution of suspended medication for more accurate dosing. The products are buffered to a pH of 4.2 and have low osmolality, along with bitterness masking. The SyrSpend SF Alka vehicles are buffered to a pH of greater than 7.7
REFERENCES
1. U.S. Pharmacopeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; July 2021.
2. Polonini HC, Silva SL, Cunha CN, et al. Compatibility of cholecalciferol, haloperidol, imipramine hydrochloride, levodopa/carbidopa, lorazepam, minocycline hydrochloride, tacrolimus monohydrate, terbinafine, tramadol hydrochloride and valsartan in SyrSpend SF PH4 oral suspensions. Pharmazie. 2016;71(4):185-191.
3. Allen LV Jr. Summary of quality-control testing for sterile and nonsterile compounded preparations, part 1: physical and chemical testing. IJPC. 2019;23(3):211-216.
4. Allen LV Jr. Summary of quality-control testing for sterile and nonsterile compounded preparations, part 2: microbiological testing. IJPC. 2019;23(4):299-303.
5. RxList. Tofranil. www.rxlist.com/tofranil-drug.htm. Accessed June 26, 2021.
6. Fagron. Product innovations: SyrSpend SF pH4. https://fagron.com/en/product-innovations/syrspend%C2%AE-sf. Accessed June 26, 2021.
7. Fagron. SyrSpend SF family of products. https://us.fagron.com/en-us/syrspend-sf-family-products. Accessed June 26, 2021.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





