US Pharm. 2021;46(12):31-39.
ABSTRACT: Very little data are available on how the coronavirus disease 2019 (COVID-19) pandemic will affect influenza, and although new information is emerging daily, much remains to be learned. Infection-control measures undertaken as a result of the COVID-19 pandemic have significantly impacted the annual influenza season, with a substantial drop in positive influenza cases compared with previous years. Despite the lack of influenza circulation, data suggest that coinfection with influenza and severe acute respiratory syndrome coronavirus 2 worsens disease severity and worsens prognosis. Although the spread of influenza decreased, misinformation has widely increased. Pharmacists have proven to be essential in the community, offering support not only in vaccine administration and point-of-care testing but also in combating misinformation through education.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the resultant coronavirus disease 2019 (COVID-19) have captured the world’s attention for the past 2 years. The COVID-19 pandemic has significantly impacted every country, industry, and person while irreparably changing not only healthcare but life on a global scale. The influenza virus typically disrupts life each season, and despite extensive research it remains responsible for thousands of deaths per season and has caused multiple pandemics. However, very little data are available on how the COVID-19 pandemic will affect influenza, and although new information is emerging daily, much remains to be learned. The objective of this article is to provide a general review of the effect the COVID-19 pandemic has had on seasonal influenza.
Background
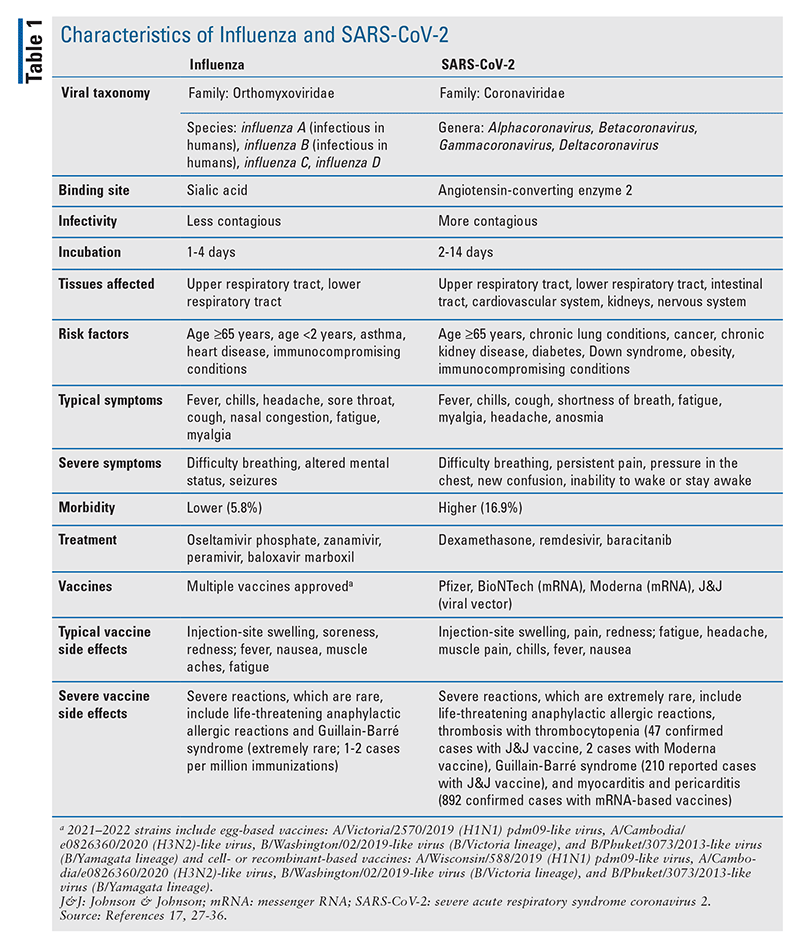
Compared with SARS-CoV-2, influenza has circulated for hundreds of years and has caused multiple pandemics.1 The 1918–1919 influenza pandemic, also known as the “Spanish flu,” infected more than 500 million people and caused an estimated 50 million deaths, whereas the most recent H1N1 influenza pandemic (in 2009) caused an estimated 500,000 deaths worldwide.2 From 2010 to 2020, an estimated 9 million to 45 million influenza cases per year, with 12,000 to 61,000 deaths, have been reported.3 On December 12, 2019, initial cases of what came to be known as COVID-19 were identified in Wuhan, China; not long thereafter, on March 11, 2020, COVID-19 was declared a pandemic by the World Health Organization.4 In circumnavigating the globe, COVID-19 has resulted in more than 256 million cases and 5.1 million deaths as of November 22, 2021.5 TABLE 1 compares the characteristics of influenza and SARS-CoV-2.
COVID-19’s Impact on Influenza
Infection-control measures undertaken during the COVID-19 pandemic have significantly impacted the annual influenza season. The 2019–2020 influenza season was considered a moderate one, with roughly 38 million cases and 22,000 deaths.3 In March 2020, around the same time that mask mandates and stay-at-home orders were enacted, a reduction in influenza infections occurred. Although infection-control measures may have influenced this decrease, the typical influenza season lasts until May, and the drop in influenza infections may have been due to the arrival of the end of the season.
The 2020–2021 influenza season saw a drastic drop in both positive influenza cases and the rate of hospitalizations compared with previous influenza seasons. Of the 1,081,671 clinical samples tested for influenza between 2020 and 2021, only 1,899 samples were positive for either influenza A or influenza B.6 This is a considerable change from previous influenza seasons: An estimated 38 million people were infected with influenza in the 2019–2020 season, and 35.5 million were infected in the 2018–2019 season.3 The rate of influenza-associated hospitalizations for the 2020–2021 season was the lowest ever reported, with 0.8 influenza-associated hospitalizations per 100,000 persons infected.7 Comparatively, in the past 10 years, the rate of influenza-associated hospitalizations per 100,000 persons infected varied widely, from 8.7 in 2011–2012 to 102.9 in 2017–2018 to 66.2 in 2019–2020.7 The drastic reduction in positive influenza cases and hospitalizations may be attributable to the increased infection-control measures used worldwide because of the COVID-19 pandemic.
The 2020–2021 influenza season also saw an increase in the number of influenza vaccines distributed.8 During the 2020–2021 influenza season, 193.8 million influenza vaccines were distributed, more than in any single previous influenza season. In the past 20 years, the number of influenza vaccines distributed ranged from 57 million during the 2004–2005 season to 174.5 million during the 2019–2020 season.
With significant infection-control measures in place and influenza vaccine administration increased, the 2020–2021 influenza season was all but nonexistent. From September 2020 through May 2021, only 0.2% (1,675/818,393) of evaluated respiratory specimens were positive for an influenza virus.6 Although social distancing and mask mandates may have eliminated the 2020–2021 influenza season, COVID-19 persists, underscoring its significant infectivity and the need for increased infection-control measures and vaccine acceptance.
Coinfection With Influenza and COVID-19
Despite the lack of influenza circulation during the 2020–2021 influenza season, data suggest that coinfection with influenza and SARS-CoV-2 impacts disease severity. Current evidence is primarily based on cell studies, animal models, and retrospective studies. Interestingly, human alveolar basal epithelial cells infected with influenza A virus have a threefold increase in expression of angiotensin-converting enzyme 2. This enzyme is the receptor in which the spike protein of the SARS-CoV-2 virus binds for entry into a host cell.9
Compared with cells infected only with SARS-CoV-2, cells preinfected with influenza A virus increased SARS-CoV-2 infectivity more than fivefold, whereas cells preinfected with another respiratory virus (i.e., respiratory syncytial virus, parainfluenza, or rhinovirus 3) showed no change.9 Mice with SARS-CoV-2 preinfected with influenza A virus had significantly higher SARS-CoV-2 viral loads and greater lung damage compared with mice without influenza A virus preinfection.9 Research in hamsters preinfected with SARS-CoV-2 or influenza A virus, either together or sequentially, showed that coinfected hamsters had significantly more weight loss, severe lung damage, higher cytokine expression, and a longer clinical course compared with those infected with a single virus.10 Moreover, hamsters initially infected with influenza A virus and then with SARS-CoV-2 virus demonstrated lower SARS-CoV-2 viral loads but greater lung damage than those infected with SARS-CoV-2 virus alone. Additionally, hamsters initially infected with influenza A virus and then with SARS-CoV-2 virus had higher influenza A viral loads than hamsters infected with influenza A virus alone.10 Therefore, coinfection with influenza A virus appears to augment lung damage caused by SARS-CoV-2 alone, and if it is established before SARS-CoV-2, influenza A appears to enhance the disease.
Early data in humans shows that coinfection may worsen prognosis. A review of 307 patients with SARS-CoV-2 infection hospitalized in Wuhan, China, from January through February 2020 showed that 49.8% were coinfected with influenza A virus and 7.5% with influenza B virus.11 Patients coinfected with influenza B virus were more likely to have severe disease characterized by fatigue upon presentation, abnormal chest CT, and decreased lymphocytes and eosinophils. Furthermore, patients with influenza B virus coinfection were more likely to present with a poor prognosis (30.4%) compared with patients with SARS-CoV-2 only (7.6%) or coinfection with influenza A virus (5.9%).
According to the National Institutes of Health guideline for management of COVID-19 and current CDC guidelines, hospitalized patients with acute respiratory illnesses should be tested for both SARS-CoV-2 and influenza viruses when both viruses are circulating.12,13 However, testing for influenza A virus in outpatients with acute respiratory illness is recommended only if the results will change clinical management.
The Pharmacist’s Role
Pharmacists are essential for combating infectious disease and providing support throughout each influenza season and the current COVID-19 pandemic. Consistently rated the most accessible healthcare professionals, pharmacists have a significant presence in the community, with more than 88,000 community pharmacies across the United States.14 Therefore, pharmacists are well positioned to assist the population by providing fact-based education, infectious-disease testing, and vaccine distribution throughout each influenza season as well as the current pandemic.
Education Centers: The COVID-19 pandemic has been a significant target for misinformation, with everything from household cleaners to vitamin C being suggested as cures.15 However, even prior to the pandemic misinformation was common, and various home remedies, such as elderberry syrup and essential oils, have been touted as cures for influenza. Fact-based education can be readily supplied by pharmacists, who serve as an information resource accessible to most patients, as more than 90% of people live within 5 miles of a pharmacy.16
Answering patients’ questions will continue to be a major role of the everyday pharmacist, and that is especially true this influenza season. Pharmacists must be prepared to answer any questions that patients may pose. Some common questions and answers include the following:
Patient: Can I get the flu vaccine and the COVID-19 vaccine at the same time?
Pharmacist: Yes. Current CDC recommendations allow the COVID-19 vaccine to be administered regardless of other recent immunizations. It is recommended that the vaccines be given in opposite arms, as more muscle pain may occur if the same arm is used for both.17
Patient: If the flu was nonexistent last year, is it necessary to get the flu vaccine this year?
Pharmacist: Yes. Influenza will circulate again this year, and it may also increase complications of COVID-19.17
Patient: Will medications such as Tamiflu work for COVID-19?
Pharmacist: No. Oseltamivir (Tamiflu) inhibits a compound used specifically by influenza and other viruses to release the newly formed virus from an infected cell so it can spread throughout the body. COVID-19 does not use the same compound for its release.18
Point-of-Care Testing: Point-of-care testing (POCT) gives pharmacists the ability to actively engage with patients and promote the direct-patient-care aspect of pharmacy.19 Pharmacies offering POCT must obtain a clinical laboratory improvements amendment (CLIA) waiver from the Centers for Medicare & Medicaid Services (CMS).20 Although pharmacists cannot bill CMS for time or services as a heathcare provider, a CLIA waiver allows them to bill CMS for their time and service in administering specific tests, such as that for COVID-19.20,21 Several products are available for rapid detection of COVID-19 and/or influenza A and B viruses via POCT. In 2019, only 12,157 pharmacies were eligible to administer POCT; in August 2021, the number nearly doubled (23,689 pharmacies).22 POCT services will remain critically important throughout the current and future influenza seasons.
POCT services may also open the door to increased protocol-based, prescriptive authority for pharmacists.19 Depending on specific state regulations, it may be possible for pharmacists to test patients for influenza and, if positive, to prescribe oseltamivir based on a protocol. Certain states already allow pharmacists to participate in collaboration agreements and to prescribe medications such as HIV preexposure prophylaxis provided that the patient has a negative HIV test, with POCT eliminating the need for a separate laboratory visit.
Vaccine Distribution: Pharmacists are trusted healthcare professionals with specific training enabling them to counsel and administer immunizations. Pharmacists have been performing immunizations for nearly three decades; the first formal immunization training took place in 1994.23 During the 2020–2021 influenza season, more than 48 million influenza vaccines were administered in pharmacies.24 On February 11, 2020, the U.S. government tapped pharmacists’ accessibility and experience by initiating the federal community-pharmacy program for COVID-19 vaccination with the purpose of providing access to COVID-19 vaccines.25 Following the CDC’s recommendation for a booster dose in high-risks persons, community pharmacists administered more than 400,000 booster doses over the course of one weekend.26 The program has proved successful, with more than 162.8 million COVID-19 doses administered by community pharmacies as of November 9, 2021.25
Conclusion
Each year, influenza puts significant strain on the healthcare system and causes thousands of deaths. However, given the impact of the current COVID-19 global pandemic and increased infection-control measures, the 2020–2021 influenza season was nearly eliminated. Therefore, it is essential that all healthcare professionals prepare for the increased burden that cocirculation of influenza and SARS-CoV-2 may cause. It is important for pharmacists to promote trust in science and to provide fact-based information in order to encourage immunization and help protect patients from the damage influenza may cause in this new COVID-19 world.
REFERENCES
1. Potter CW. A history of influenza. J Appl Microbiol. 2001;91(4):572-579.
2. CDC. History of 1918 flu pandemic. www.cdc.gov/flu/pandemic-resources/1918-commemoration/1918-pandemic-history.htm?web=1&wdLOR=cCA07C6BA-7D2A-404B-8A44-B17501ED95F4. Accessed September 20, 2021.
3. CDC. Disease burden of flu. www.cdc.gov/flu/about/burden/index.html. Accessed September 20, 2021.
4. World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19—11 March 2020. www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Accessed September 25, 2021.
5. World Health Organization. WHO coronavirus (COVID-19) dashboard. https://covid19.who.int. Accessed November 1, 2021.
6. CDC. 2020-2021 flu season summary. www.cdc.gov/flu/season/faq-flu-season-2020-2021.htm#anchor_1627000324276. Accessed September 20, 2021.
7. CDC. Influenza Hospitalization Surveillance Network (FluSurv-NET). www.cdc.gov/flu/weekly/influenza-hospitalization-surveillance.htm. Accessed September 21, 2021.
8. CDC. Historical reference of seasonal influenza vaccine doses distributed. www.cdc.gov/flu/prevent/vaccine-supply-historical.htm?web=1&wdLOR=cFBD27FBD-6D5F-BD45-841E-3F4931E6AEA7. Accessed September 23, 2021.
9. Bai L, Zhao Y, Dong J, et al. Coinfection with influenza A virus enhances SARS-CoV-2 infectivity. Cell Res. 2021;31(4):395-403.
10. Zhang AJ, Lee AC, Chan JF, et al. Coinfection by severe acute respiratory syndrome coronavirus 2 and influenza A(H1N1)pdm09 virus enhances the severity of pneumonia in golden Syrian hamsters. Clin Inf Dis. 2021;72(12):e978-e992.
11. Yue H, Zhang M, Xing L, et al. The epidemiology and clinical characteristics of co-infection of SARS-CoV-2 and influenza viruses in patients during COVID-19 outbreak. J Med Virol. 2020;92(11):2870-2873.
12. National Institutes of Health. COVID-19 treatment guidelines: what’s new in the guidelines. www.covid19treatmentguidelines.nih.gov/about-the-guidelines/whats-new/. Accessed September 21, 2021.
13. CDC. Testing guidance for clinicians when SARS-CoV-2 and influenza viruses are co-circulating. www.cdc.gov/flu/professionals/diagnosis/testing-guidance-for-clinicians.htm. Accessed September 30, 2021.
14. IQVIA. U.S. national pharmacy market summary. www.onekeydata.com/downloads/reports/IQVIA_Report_US_Pharmacy_Market_Report_2019.pdf. Accessed November 15, 2021.
15. Erku DA, Belachew SA, Abrha S, et al. When fear and misinformation go viral: pharmacists’ role in deterring medication misinformation during the ‘infodemic’ surrounding COVID-19. Res Social Adm Pharm. 2021;17(1):1954-1963.
16. CDC. Get to know your pharmacist. www.cdc.gov/heartdisease/pharmacist.htm. Accessed September 25, 2021.
17. CDC. COVID-19 ACIP vaccine recommendations. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html. Accessed September 25, 2021.
18. Tamiflu (oseltamivir) package insert. South San Francisco, CA: Genentech, Inc; August 2019.
19. Klepser NS, Klepser DG, Adams JL, et al. Impact of COVID-19 on prevalence of community pharmacies as CLIA-waived facilities. Res Social Adm Pharm. 2021;17(9):1574-1578.
20. CDC. Waived tests. www.cdc.gov/labquality/waived-tests.html. Accessed September 30, 2021.
21. American Society of Health-System Pharmacists. Provider status. www.ashp.org/advocacy-and-issues/provider-status?loginreturnUrl=SSOCheckOnly. Accessed September 30, 2021.
22. Division of Clinical Laboratory Improvement and Quality, Centers for Medicare & Medicaid Services. Laboratories by type of activity. www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/Downloads/factype.pdf. Accessed September 30, 2021.
23. Hogue MD, Grabenstein JD, Foster SL, Rothholz MC. Pharmacist involvement with immunizations: a decade of professional advancement. J Am Pharm Assoc (2003). 2006;46(2):168-179.
24. CDC. Influenza vaccinations administered in pharmacies and physician medical offices, United States. www.cdc.gov/flu/fluvaxview/dashboard/vaccination-administered.html. Accessed September 25, 2021.
25. CDC. The Federal Retail Pharmacy Program for COVID-19 vaccination. www.cdc.gov/vaccines/covid-19/retail-pharmacy-program/index.html. Accessed November 1, 2021.
26. White House. Press briefing by White House COVID-19 response team and public health officials. www.whitehouse.gov/briefing-room/press-briefings/2021/09/28/press-briefing-by-white-house-covid-19-response-team-and-public-health-officials-58/. Accessed September 30, 2021.
27. Dou D, Revol R, Östbye H, et al. Influenza A virus cell entry, replication, virion assembly and movement. Front Immunol. 2018;9:1581.
28. Huang Y, Yang C, Xu XF, et al. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41(9):1141-1149.
29. Scudellari M. How the coronavirus infects cells—and why Delta is so dangerous. Nature. 2021;595(7869):640-644.
30. Solomon DA, Sherman AC, Kanjilal S. Influenza in the COVID-19 era. JAMA. 2020;324(13):1342-1343.
31. Flerlage T, Boyd DF, Meliopoulos V, et al. Influenza virus and SARS-CoV-2: pathogenesis and host responses in the respiratory tract. Nat Rev Microbiol. 2021;19(7):425-441.
32. Piroth L, Cottenet J, Mariet AS, et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021;9(3):251-259.
33. CDC. Flu symptoms & complications. www.cdc.gov/flu/symptoms/symptoms.htm. Accessed September 30, 2021.
34. CDC. Symptoms of COVID-19. www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed September 27, 2021.
35. CDC. Different COVID-19 vaccines. www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html. Accessed September 30, 2021.
36. CDC. Selected adverse events reported after COVID-19 vaccination. www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html. Accessed September 30, 2021.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






