US Pharm. 2024;49(2):HS6-HS12.
ABSTRACT: Rheumatoid arthritis (RA), which affects approximately 1% of Americans, is a systemic inflammatory autoimmune disease that has both articular and extraarticular manifestations. RA patients are at greater risk for major adverse cardiovascular events (MACE) and venous thromboembolism (VTE). Janus kinase inhibitors (JAKi) are an effective treatment for RA; however, their use in certain RA patient populations (e.g., smokers aged >65 years) potentiates the risk of adverse events. Pharmacists play a crucial role in informing RA patients about the risk factors associated with JAKi, the warning signs and symptoms of MACE and VTE, and the benefits of smoking cessation.
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by chronic inflammation that has both articular (synovial joints, cartilage, and bone) and extra-articular (vasculature, eyes, mouth, skin, lungs, and heart) manifestations. Although RA primarily affects the synovial joints, patients with extra-articular involvement may experience severe complications—such as interstitial lung disease and cardiovascular disease (CVD)—that can worsen the overall prognosis.1,2 Approximately 1.3 million Americans (0.6%-1.0% of the population) have RA, with women primarily being affected.3,4 Although the cause of RA is unknown, risk factors include middle age (ages 40-60 years), family history of RA, smoking, obesity, and exposure to air pollution. Early symptoms of RA include tenderness, pain, and swelling of the joints that can lead to joint dysfunction if untreated. In addition to joint inflammation, some patients experience malaise, fatigue, and low-grade fever, as well as disease flares that can last days or weeks.5,6
RA patients have an increased risk of CVD. The abnormal lipid metabolism, chronic inflammation, and endothelial dysfunction seen in RA can result in CVD, the leading cause of death in RA patients.7,8 In a phenomenon known as the lipid paradox, the CVD risk is greater despite low levels of total cholesterol (TC), LDL, and HDL. Whereas HDL in the optimal range is cardioprotective, the low HDL levels occurring in RA contribute to poor antioxidant capacity and a reduced anti-inflammatory state that is unable to counteract LDL oxidation, leading to an acceleration in atherosclerosis.8-10 Moreover, increased levels of inflammatory cytokines (e.g., interleukin [IL]-1, IL-6, tumor necrosis factor [TNF]-alpha), which are typically elevated in RA patients, are associated with CVD.2,11
Compared with the general population, RA patients have twice the risk of developing venous thromboembolism (VTE), including deep-vein thrombosis (DVT) and pulmonary embolism (PE). Increased disease activity in RA patients is associated with DVT risk and, to a greater extent, PE risk.12 This increase in risk is due to inflammation-induced endothelial cell dysfunction, stasis, and hypercoagulability (i.e., Virchow’s triad), which contribute to a prothromboembolic state.13 In addition, the proinflammatory cytokines IL-17 and TNF-alpha, which are higher in RA patients, can promote procoagulant and prothrombic effects in the vasculature.14 This prothrombotic state may be enhanced by additional risk factors, such as immobilization, surgery, hormone therapy, obesity, or comorbidities (e.g., hypertension, diabetes, malignancy).12,15
Multiple inflammatory cytokines and signaling pathways have been implicated in the etiology and pathogenesis of RA. Activation of one key pathway—the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway—is involved in both RA and CVD. JAK/STAT signaling leads to increased levels of proinflammatory cytokines that mediate disease flares and progression in RA.16,17
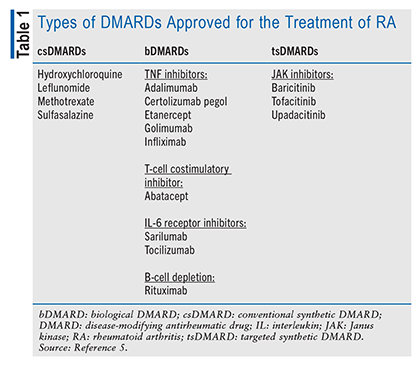
Current treatments to halt joint damage and disease progression include both nonpharmacologic methods (reduction of joint stress, physical and occupational therapy, and surgical intervention) and pharmacologic therapies (nonsteroidal anti-inflammatory drugs [NSAIDs], glucocorticoids, and disease-modifying antirheumatic drugs [DMARDs]). NSAIDs and/or glucocorticoids, which are frequently used in conjunction with DMARDs, also can increase a patient’s CVD risk.18 There are three categories of DMARDs: conventional synthetic DMARDs (csDMARDs), biological DMARDs (bDMARDs), and targeted synthetic DMARDs (tsDMARDs) (TABLE 1). The first-line therapy for RA is methotrexate, with remission being the treatment goal. If remission is not achieved after therapy with two csDMARDs and there is high disease activity, then bDMARDs or tsDMARDs (e.g., JAK inhibitors [JAKi]) may be employed.5,19 In addition to the existing increased risk of CVD and VTE in RA, JAKi use confers an increased risk, according to updated black box warnings from the FDA.20-22 This article will review the current evidence for CVD and VTE risk in RA patients treated with JAKi and discuss appropriate patient education for the safe use of JAKi.5,19
JAKi Overview
JAKi are used to treat various chronic inflammatory disorders—such as RA, psoriatic arthritis (PsA), ulcerative colitis, and atopic dermatitis—that may involve continuous or increased activation of the JAK/STAT signaling pathway.17 JAKs are dimerized enzyme-linked receptors that transmit extracellular signals intracellularly through phosphorylation and dimerization of STATs to induce gene transcription pathways. This leads to the production of cytokines, chemokines, and growth factors that are involved in coordinating the function of immune cells.23 JAK/STAT signaling is complex because of the multiple JAK and tyrosine kinase (TYK) receptor combinations (e.g., JAK1/JAK2, JAK1/JAK3, JAK2/TYK2). The JAK/STAT pathway is activated when cytokines (e.g., IL-2, IL-6, IL-10, interferon-gamma) bind to these extracellular receptors.24 Normally, suppressors of cytokine signaling and protein inhibitors of activated STAT proteins impede JAK/STAT signaling; however, in RA, these regulators are dysfunctional.17 Elevated levels of IL-6 and IL-10—cytokines generated when the JAK/STAT pathway is activated—are involved in the dyslipidemia occurring in chronic inflammatory disorders.25,26 Inhibiting the JAK/STAT signaling pathway improves therapeutic outcomes partly by reducing disease activity; however, whether selective inhibition of the JAK/STAT pathway reduces adverse effects is still being evaluated.20
The oral JAKi approved for RA are tofacitinib (Xeljanz), upadacitinib (Rinvoq), and baricitinib (Olumiant). These agents differentially target the JAK/STAT signaling pathway to suppress cytokine signaling. Tofacitinib, a first-generation pan-JAKi, targets JAK1/JAK2, JAK1/JAK3, and JAK2/JAK2, leading to decreased T-cell differentiation and inflammatory cytokine production.22,24 Baricitinib, another first-generation JAKi, primarily targets JAK1- and JAK2-mediated signaling, resulting in reduced leukocyte recruitment and inflammatory-cytokine production.21,24 Upadacitinib, a second-generation JAKi, selectively targets JAK1, leading to a decrease in IL-6 and IL-7.20,24 To date, no trials have compared efficacy among these JAKi; however, a meta-analysis of clinical-trial data found that when each DMARD was combined with methotrexate, baricitinib (4 mg) and upadacitinib (15 mg) were superior to the TNF inhibitor (TNFi) adalimumab (40 mg) in achieving 70% improvement in the number of tender and swollen joints and markers of inflammation in RA patients.27
JAKi class effects include increased infection risk, cytopenia, and lipid abnormalities. In addition, JAKi should be used with caution in patients with a history of diverticulitis or NSAID use owing to the increased risk of gastrointestinal (GI) perforations.20-22,27 Other common adverse effects include upper respiratory infections, urinary tract infections, nasopharyngitis, nausea, headaches, and occasional diarrhea.28 Black box warnings note an increased risk of serious events, such as serious infections, neoplasms, major adverse CV events (MACE), thrombosis, and death.29,30
Specifically, JAKi use is strongly associated with lipid abnormalities, resulting in increased levels of TC, LDL, HDL, apolipoprotein (Apo) A1, and ApoB. These aberrant lipid levels are typically observed within the first 4 weeks and ultimately stabilize by 12 weeks of treatment.31 The underlying mechanism partly involves dysfunctional cholesterol ester metabolism and impaired antioxidant capacity despite elevated HDL levels.9 JAKi may also reverse the lipid paradox, thereby restoring or increasing lipid levels in RA patients.32 Given that RA patients are susceptible to adverse CV events, these JAKi-induced phenomena further contribute to the development of MACE and VTE.33,34 Although European RA guidelines reflect this risk by recommending that JAKi be reserved for patients refractory to first-line DMARDs who have been screened for associated risk factors, the American College of Rheumatology (ACR) guidelines do not state a preference between bDMARDs or tsDMARDs.5,19 However, the ACR guidelines may be revised following the evaluation of recently published JAKi safety data.
JAKi-Associated CV and VTE Risk
Although drug efficacy and survival are similar between JAKi and TNFi, an increased risk of adverse CV events has been reported in RA patients treated with JAKi.35,36 A plethora of trial data and post hoc analyses examining the safety profile of JAKi has recently been published. Although many DMARDs, such as methotrexate and TNFi, have cardioprotective effects in RA patients, recent data suggest that JAKi use may increase the risk of MACE and VTE.2,18
In response to increased serum lipid levels and malignancy reported in clinical trials of tofacitinib, the FDA and the European Medicines Agency required postmarketing trials to further investigate drug safety.29,30 The resulting Oral Rheumatoid Arthritis Trial Surveillance phase IIIb/IV randomized, open-label, noninferiority trial was conducted to determine incidence rates of MACE and malignancy in RA patients aged >50 years with one or more CV risk factors (e.g., smoking, hypertension, low HDL, diabetes, history of coronary artery disease or coronary heart disease, extraarticular disease) who were treated with tofacitinib (5 mg or 10 mg) or a TNFi (adalimumab or etanercept). Both tofacitinib groups had a higher incidence of MACE compared with the TNFi group, and incidence rates were higher in patients aged >65 years.30,34
Subsequent post hoc analyses sought to determine the role of two independent risk factors (age and smoking status) by comparing a high-risk group (RA patients aged >65 years with current or history of smoking) and a low-risk group (aged <65 years with no smoking history). The relative risk of MACE and VTE was elevated in high-risk patients receiving tofacitinib compared with high-risk TNFi-treated patients, and no difference was observed in the low-risk group.37,38 Moreover, no difference was detected between tofacitinib and TNFi in low-risk patients with identifiable CV risk factors over a 6-year period.35 Patients who experienced a VTE had a history of hypertension and higher BMI compared with age- and treatment-matched controls.37 With baricitinib, an increased incidence of MACE and VTE was seen in RA patients aged >65 years with one or more CV risk factors compared with younger RA patients who did not have CV risk factors.39
These studies primarily involved white patients; however, a real-world retrospective, observational study in Korean patients with RA showed a similar overall risk of MACE and VTE between patients treated with JAKi (tofacitinib or baricitinib) and those treated with TNFi, suggesting that results may differ by ethnicity.40 No differences in MACE incidence rates were observed between upadacitinib, adalimumab, and methotrexate in RA and PsA patients.41
RA patients have an increased incidence of VTE compared with the general population; the incidence is higher in men and increases with age.12 An elevated incidence of VTE with tofacitinib use was observed in patients with immune-mediated inflammatory conditions (RA, psoriasis, and PsA) compared with adalimumab-treated patients.15 PE and DVT incidence rates were greater in RA patients with CV risk factors.15 In a Swedish longitudinal clinical register study, the VTE risk—predominantly PE risk—in RA patients treated with a JAKi (tofacitinib or baricitinib) was double that of RA patients treated with a TNFi or a non-TNF bDMARD and triple that of the general population.42 A U.S.-based observational study showed a higher but nonsignificant risk of VTE in JAKi-naïve versus TNFi bDMARD-naïve RA patients.43 In a long-term safety study, no differences in VTE incidence rates were observed in groups of RA patients receiving upadacitinib, adalimumab, or methotrexate.41
Although much of the recent data suggest that high-risk RA patients (aged ≥65 years and current or former smokers) have an increased risk of MACE and VTE, more long-term studies are needed to determine the risk profile of JAKi. Nonetheless, these patients should be screened for CVD and VTE risk factors prior to initiating JAKi therapy.
The Pharmacist’s Role
Pharmacists serve as a last-line resource to improve medication adherence and overall health outcomes by providing key information and counseling points to their patients. Considering that JAKi carry multiple black box warnings, the following counseling points should be discussed prior to treatment initiation and on subsequent refill requests.
VTE (DVT/PE) Counseling: Clinical studies indicate that JAKi increase the risk of thrombosis, particularly PE, which can lead to stroke or heart attack. Advanced age, obesity, immobilization, surgery, and comorbidities such as infection, chronic obstructive pulmonary disease, hyperlipidemia, and hypertension are risk factors for VTE.12,44 Patients should be counseled on the signs and symptoms of PE, such as chest pain, shortness of breath, tachypnea, and tachycardia. DVT is another possible complication, and patients should be advised to watch out for the development of unilateral leg pain, erythema, and/or swelling.45 Patients experiencing these symptoms should seek immediate medical help and notify the healthcare team of their JAKi use.
Smoking Cessation: JAKi pose a greater risk of MACE and VTE in patients who are current or former smokers.38 Pharmacists can assist with smoking-cessation products and support. Also, smokers using hormonal contraceptives or undergoing hormone replacement therapy are at increased risk for VTE.46
Vaccinations: RA patients treated with JAKi have an increased risk of herpes zoster infection.30 To reduce the risk of infections, pharmacists can play an important role in immunizing patients on JAKi therapy. Patients should be instructed to hold the use of JAKi for 1 week before and 4 weeks after vaccination with a live-attenuated virus vaccine.47 Non–live-attenuated vaccines, however, may be administered while the patient is receiving JAKi therapy.47
Other Adverse Effects: Patients treated with JAKi are also at greater risk for malignancies. Cancer subtypes of interest include breast cancer (females only), lymphoma, lung cancer (including non–small-cell and small-cell lung cancers), melanoma, prostate cancer, colorectal cancer, and pancreatic cancer.48 Individuals who have a history of diverticulitis or are undergoing concurrent treatment with NSAIDs and/or glucocorticoids are more susceptible to GI perforation.20-22 Therefore, these patients should be counseled to monitor for new-onset abdominal pain or other GI symptoms.
Key Laboratory Values: Alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and bilirubin should be assessed at baseline, 4 to 8 weeks, and every 3 months thereafter. TC, LDL, HDL, and triglycerides should be monitored at baseline, 8 to 12 weeks, and periodically thereafter. Elevation usually occurs within the first 4 to 12 weeks of therapy.49 A CBC with differential should be performed at baseline and every 3 months thereafter to monitor for lymphopenia, neutropenia, and anemia.22 Patients may experience fatigue, and they may be at greater risk for infection. Caution should be used in large group settings, exposure to persons who are ill should be minimized, and routine handwashing should be practiced.
Conclusion
JAKi have been shown to reduce disease activity in patients with RA. However, recent data suggest that treatment guidelines should be updated to recommend that patients who are at higher risk for MACE and VTE because of their age and smoking status be screened prior to initiating JAKi therapy. Pharmacists play a vital role in assisting RA patients receiving JAKi therapy by addressing their concerns, counseling them on the signs and symptoms of VTE, promoting health through vaccinations, and providing smoking-cessation support.
REFERENCES
1. Mori S. Management of rheumatoid arthritis patients with interstitial lung disease: safety of biological antirheumatic drugs and assessment of pulmonary fibrosis. Clin Med Insights Circ Respir Pulm Med. 2015;9(Suppl 1):1-49.
2. Giachi A, Cugno M, Gualtierotti R. Disease-modifying anti-rheumatic drugs improve the cardiovascular profile in patients with rheumatoid arthritis. Front Cardiovasc Med. 2022;9:1012661.
3. Gerosa M, De Angelis V, Riboldi P, Meroni PL. Rheumatoid arthritis: a female challenge. Womens Health (Lond). 2008;4(2):195-201.
4. Maranini B, Bortoluzzi A, Silvagni E, Govoni M. Focus on sex and gender: what we need to know in the management of rheumatoid arthritis. J Pers Med. 2022;12(3):499.
5. Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2021;73(7):924-939.
6. Tanaka Y. Rheumatoid arthritis. Inflamm Regen. 2020;40:20.
7. England BR, Thiele GM, Anderson DR, Mikuls TR. Increased cardiovascular risk in rheumatoid arthritis: mechanisms and implications. BMJ. 2018;361:k1036.
8. Kahlenberg JM, Kaplan MJ. Mechanisms of premature atherosclerosis in rheumatoid arthritis and lupus. Annu Rev Med. 2013;64:249-263.
9. Yan J, Yang S, Han L, et al. Dyslipidemia in rheumatoid arthritis: the possible mechanisms. Front Immunol. 2023;14:1254753.
10. Erum U, Ahsan T, Khowaja D. Lipid abnormalities in patients with rheumatoid arthritis. Pak J Med Sci. 2017;33(1):227-230.
11. Amin MN, Siddiqui SA, Ibrahim M, et al. Inflammatory cytokines in the pathogenesis of cardiovascular disease and cancer. SAGE Open Med. 2020;8:2050312120965752.
12. Molander V, Bower H, Frisell T, Askling J. Risk of venous thromboembolism in rheumatoid arthritis, and its association with disease activity: a nationwide cohort study from Sweden. Ann Rheum Dis. 2021;80(2):169-175.
13. Ketfi C, Boutigny A, Mohamedi N, et al. Risk of venous thromboembolism in rheumatoid arthritis. Joint Bone Spine. 2021;88(3):105122.
14. Yang X, Chang Y, Wei W. Endothelial dysfunction and inflammation: immunity in rheumatoid arthritis. Mediators Inflamm. 2016;2016:6813016.
15. Mease P, Charles-Schoeman C, Cohen S, et al. Incidence of venous and arterial thromboembolic events reported in the tofacitinib rheumatoid arthritis, psoriasis and psoriatic arthritis development programmes and from real-world data. Ann Rheum Dis. 2020;79(11):1400-1413.
16. Ciobanu DA, Poenariu IS, Crînguș LI, et al. JAK/STAT pathway in pathology of rheumatoid arthritis (review). Exp Ther Med. 2020;20(4):3498-3503.
17. Malemud CJ. The role of the JAK/STAT signal pathway in rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2018;10(5-6):117-127.
18. Ozen G, Pedro S, Michaud K. The risk of cardiovascular events associated with disease-modifying antirheumatic drugs in rheumatoid arthritis. J Rheumatol. 2021;48(5):648-655.
19. Smolen JS, Landewé RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023;82(1):3-18.
20. Rinvoq (upadacitinib) product information. North Chicago, IL: AbbVie Inc; November 2023.
21. Olumiant (baricitinib) product information. Indianapolis, IN: Lilly USA, LLC; June 2022.
22. Xeljanz (tofacitinib) product information. New York, NY: Pfizer Inc; December 2021.
23. Morinobu A. JAK inhibitors for the treatment of rheumatoid arthritis. Immunol Med. 2020;43(4):148-155.
24. Hu X, Li J, Fu M, et al. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther. 2021;6(1):402.
25. Moraitis AG, Freeman LA, Shamburek RD, et al. Elevated interleukin-10: a new cause of dyslipidemia leading to severe HDL deficiency. J Clin Lipidol. 2015;9(1):81-90.
26. Pietrzak A, Chabros P, Grywalska E, et al. Serum concentration of interleukin 6 is related to inflammation and dyslipidemia in patients with psoriasis. Postepy Dermatol Alergol. 2020;37(1):41-45.
27. Harrington R, Al Nokhatha SA, Conway R. JAK inhibitors in rheumatoid arthritis: an evidence-based review on the emerging clinical data. J Inflamm Res. 2020;13:519-531.
28. Cohen S, Reddy V. Overview of the Janus kinase inhibitors for rheumatologic and other inflammatory disorders. UpToDate. Waltham, MA: UpToDate Inc. www.uptodate.com. Accessed December 6, 2023.
29. FDA. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death. Accessed December 1, 2023.
30. Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316-326.
31. Czókolyová M, Hamar A, Pusztai A, et al. Effects of one-year tofacitinib therapy on lipids and adipokines in association with vascular pathophysiology in rheumatoid arthritis. Biomolecules. 2022;12(10):1483.
32. Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70(3):482-487.
33. Linton MF, Yancey PG, Davies SS, et al. The role of lipids and lipoproteins in atherosclerosis. January 3, 2019. In: Feingold KR, Anawalt B, Blackman MR, et al, eds. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc; 2000–.
34. Charles-Schoeman C, Fleischmann R, Davignon J, et al. Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis versus healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol. 2015;67(3):616-625.
35. Pope JE, Keystone E, Jamal S, et al. Persistence of tofacitinib in the treatment of rheumatoid arthritis in open-label, long-term extension studies up to 9.5 years. ACR Open Rheumatol. 2019;1(2):73-82.
36. Temmoku J, Miyata M, Suzuki E, et al. Drug retention rates and the safety of Janus kinase inhibitors in elderly patients with rheumatoid arthritis. J Clin Med. 2023;12(14):4585.
37. Weitz JI, Szekanecz Z, Charles-Schoeman C, et al. Biomarkers to predict risk of venous thromboembolism in patients with rheumatoid arthritis receiving tofacitinib or tumour necrosis factor inhibitors. RMD Open. 2022;8(2):e002571.
38. Kristensen LE, Danese S, Yndestad A, et al. Identification of two tofacitinib subpopulations with different relative risk versus TNF inhibitors: an analysis of the open label, randomised controlled study ORAL Surveillance. Ann Rheum Dis. 2023;82(7):901-910.
39. Taylor PC, Bieber T, Alten R, et al. Baricitinib safety for events of special interest in populations at risk: analysis from randomised trial data across rheumatologic and dermatologic indications. Adv Ther. 2023;40(4):1867-1883.
40. Min HK, Kim H, Jeong HJ, et al. Risk of cancer, cardiovascular disease, thromboembolism, and mortality in patients with rheumatoid arthritis receiving Janus kinase inhibitors: a real-world retrospective observational study using Korean health insurance data. Epidemiol Health. 2023;45:e2023045.
41. Burmester GR, Cohen SB, Winthrop KL, et al. Safety profile of upadacitinib over 15 000 patient-years across rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and atopic dermatitis. RMD Open. 2023;9(1):e002735.
42. Molander V, Bower H, Frisell T, et al. Venous thromboembolism with JAK inhibitors and other immune-modulatory drugs: a Swedish comparative safety study among patients with rheumatoid arthritis. Ann Rheum Dis. 2023;82(2):189-197.
43. Desai RJ, Pawar A, Weinblatt ME, Kim SC. Comparative risk of venous thromboembolism in rheumatoid arthritis patients receiving tofacitinib versus those receiving tumor necrosis factor inhibitors: an observational cohort study. Arthritis Rheumatol. 2019;71(6):892-900.
44. Mori S, Ogata F, Tsunoda R. Risk of venous thromboembolism associated with Janus kinase inhibitors for rheumatoid arthritis: case presentation and literature review. Clin Rheumatol. 2021;40(11):4457-4471.
45. Michota F. Venous thromboembolism: epidemiology, characteristics, and consequences. Clin Cornerstone. 2005;7(4):8-15.
46. LaVasseur C, Neukam S, Kartika T, et al. Hormonal therapies and venous thrombosis: considerations for prevention and management. Res Pract Thromb Haemost. 2022;6(6):e12763.
47. Bass AR, Chakravarty E, Akl EA, et al. 2022 American College of Rheumatology guideline for vaccinations in patients with rheumatic and musculoskeletal diseases. Arthritis Care Res (Hoboken). 2023;75(3):449-464.
48. Curtis JR, Yamaoka K, Chen YH, et al. Malignancy risk with tofacitinib versus TNF inhibitors in rheumatoid arthritis: results from the open-label, randomised controlled ORAL Surveillance trial. Ann Rheum Dis. 2023;82(3):331-343.
49. Charles-Schoeman C, Gonzalez-Gay MA, Kaplan I, et al. Effects of tofacitinib and other DMARDs on lipid profiles in rheumatoid arthritis: implications for the rheumatologist. Semin Arthritis Rheum. 2016;46(1):71-80.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






