US Pharm. 2023;48(8):58-59.
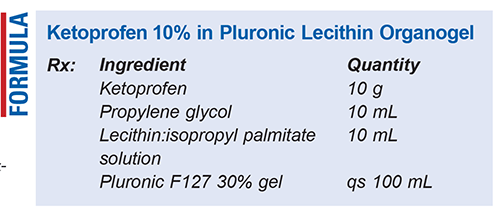
Method of Preparation: Calculate the required quantity of each ingredient for the total amount of transdermal gel to be prepared. Accurately weigh or measure each ingredient. Combine the ketoprofen powder, propylene glycol (PG), and lecithin:isopropyl palmitate solution in a suitable container and mix thoroughly until a uniform consistency is achieved. Gradually add the Pluronic F127 30% gel, mixing continuously to ensure even dispersion. Use a shear mixing method to promote homogeneity throughout the gel. Once the gel has reached final volume, package the preparation in an airtight container—taking care not to introduce air bubbles—and label.
Use: Ketoprofen has been used to treat pain and inflammation associated with osteoarthritis and rheumatoid arthritis.
Packaging: Package in a tight, light-resistant container. Store at controlled room temperature.
Labeling: Keep out of reach of children. Use only as directed. For external use only. (Note: Include any other relevant instructions or warnings on the label.)
Stability: The USP default beyond-use date for transdermal gel is up to 30 days.1
Quality Control: Weight/volume, pH, specific gravity (SG), active drug assay, color, rheologic properties/pourability, physical observation, and physical stability (discoloration, foreign materials, gas formation, mold growth) are all examples of quality-control assessments.2
Discussion: Ketoprofen (C16H14O3, MW 254.28), a nonsteroidal anti-inflammatory drug with analgesic and antipyretic properties, is effective for treating pain associated with osteoarthritis and rheumatoid arthritis. Ketoprofen occurs as a white or almost white, odorless or almost odorless, crystalline powder. It is practically insoluble in water but freely soluble in ethanol, chloroform, acetone, and ether, and its melting point is ~95°C. Ketoprofen is available as capsules and extended-release capsules. Ketoprofen should be stored in an airtight container to maintain stability.3
PG (C3H8O2) is a clear, colorless, odorless, viscous liquid with a sweet, glycerin-like taste. It is miscible with acetone, chloroform, 95% ethanol, glycerin, and water, and its SG is 1.038 g/mL. It is not miscible with fixed oils or light mineral oil. PG serves as a humectant in topicals, a preservative in solutions and semisolids, and a solvent or cosolvent in aerosols, oral solutions, parenterals, and topicals. PG may be used to carry flavors and emulsifiers. It is stable and can be mixed with other solvents. Because PG is hygroscopic, it should be stored in an airtight container and protected from light.4,5
Lecithin (egg lecithin, soybean lecithin, vegetable lecithin), which is available in semiliquid or powder form, has a bland or nutlike flavor and is brown to light yellow. It is insoluble in polar solvents and cold vegetable and animal oils but is soluble in aliphatic and aromatic hydrocarbons, mineral oil, and fatty acids. Lecithin is an effective emulsifier, stabilizer, and dispersing agent. Being high in phospholipids, lecithin is used as a component of skincare products and dietary supplements because it improves skin hydration, cell-membrane structure, and nutrient absorption.6
Isopropyl palmitate (C19H38O2, MW 298.51) is a colorless liquid with a faint odor. It is soluble in castor oil, cottonseed oil, alcohol, and mineral oil, and therefore is excellent for oil-based formulations. Its insolubility in water, glycerin, and PG makes isopropyl palmitate ideal for anhydrous products. Isopropyl palmitate is an effective emollient and improves the texture and sensory experience of skincare products and cosmetics; it also enables active ingredients to more deeply penetrate the skin.7
Poloxamer 407 (Pluronic F127), an ethylene oxide and propylene oxide block copolymer, is commonly used as an emulsifying, solubilizing, and wetting agent. It occurs as white, waxy, free-flowing granules (i.e., powder). Pluronic F127 is odorless or has a very mild odor, and it is tasteless. It has a melting point of ~56°C and is highly soluble in water, alcohol, and isopropyl alcohol.8
REFERENCES
1. U.S. Pharmacopeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; September 2022.
2. Allen LV Jr. Standard operating procedure for performing physical quality assessment of oral and topical liquids. IJPC. 1999;3:146-147.
3. Oruvail Extended-Release (ketoprofen extended-release) capsule, Extended Release Orudis (ketoprofen) capsule product information. Philadelphia, PA: Wyeth Pharmaceuticals Inc; January 2007.
4. Brayfield A, ed. Martindale: The Complete Drug Reference. 38th ed. London, England: Pharmaceutical Press; 2014:79-80, 401-107, 1905-1906.
5. Weller PJ. Propylene glycol. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. London, England: Pharmaceutical Press; 2009:592-594.
6. Shah HC, Singh KK. Lecithin. In: Sheskey PJ, Cook WG, Cable CG, eds. Handbook of Pharmaceutical Excipients. 8th ed. London, England: Pharmaceutical Press; 2017:538-541.
7. Quinn ME. Isopropyl palmitate. In: Sheskey PJ, Cook WG, Cable CG, eds. Handbook of Pharmaceutical Excipients. 8th ed. London, England: Pharmaceutical Press; 2017:497-498.
8. Pirjanian A, Alvarez-Nunez F. Poloxamer. In: Sheskey PJ, Cook WG, Cable CG, eds. Handbook of Pharmaceutical Excipients. 8th ed. London, England: Pharmaceutical Press; 2017:688-693.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






