US Pharm. 2021;46(8):39-42.
ABSTRACT: Many people experience sunburn in their lifetime. Symptoms range from redness and pain to blistered skin, headache, fever, and nausea in severe cases. Repeated overexposure to ultraviolet (UV) rays increases the risk of skin cancer, the most common form of cancer in the United States. Preventative measures include hats, sunglasses, clothing, and sunscreen. However, sunburn and the resultant skin damage still occur. Most cases of sunburn are self-limiting, and steps can be taken to mitigate the symptoms. Pharmacists play a pivotal role in educating patients regarding the risks associated with UV exposure, sunburn preventative techniques, and pharmacologic and nonpharmacologic treatment options.
Sunburn is a common condition that most people have experienced in their lifetime. The condition is caused by excessive exposure to ultraviolet radiation (UVR), which is cumulative and can lead to serious and sometimes life-threatening complications. Through education on the use of sunscreen and other avoidance or exposure-minimizing techniques, patients can reduce their risk of sunburn and the long-term complications associated with the accumulated effects of sunburns.1-3
Incidence and Prevalence
According to the Office of the Surgeon General, more than one out of every three people in the United States reports getting sunburned each year.4 The CDC estimates that at least half of all adults in the U.S. suffer from one sunburn a year, and two-thirds of those experience more than one sunburn in the same year.5-7 A March 2020 report on cancer trends in the U.S. indicated over 33,000 sunburns were reported annually, requiring emergency room visits among all racial and ethnic groups.8 Skin cancer is the most common cancer nationwide, and sunburns are associated with an increased risk of developing skin cancer.1,4,9-11
What Is Sunburn?
Sunburn is a minor, superficial burn that results in an inflammatory reaction to UVR damage to the epidermis and dermis layers of the skin.1-3,12 The radiation burn is caused by excessive UVR exposure, which causes an acute inflammatory response. Both ultraviolet A (UVA) and ultraviolet B (UVB) exposures are factors in the development of sunburn; however, UVA rays play a larger role in aging and wrinkles, while UVB rays play a more prominent role in sunburn and are responsible for directly damaging DNA.1,3,9-14 Melanin in the skin is produced faster as a protection mechanism when a person is exposed to UVR. The extra melanin leads to darkening or reddening of the skin, which is a result of the skin damage.1,12,15
This direct cellular damage, along with the activation of numerous inflammatory mediators, commonly results in erythema, edema, itching, and pain. Depending on the degree of exposure, patients may complain of fluid-filled blisters, headache, fever, nausea, and fatigue, indicating a more severe sunburn.1-3,15,16 The varying symptoms are directly proportional to the severity of the sun exposure.1-3,15 It is important to note that sunburn is a factor of the amount of UVR, not the amount of time a person is exposed to UVR.13,14
Risk Factors for Sunburn
Several risk factors are associated with an increased risk of sunburn. These include working outdoors, living or vacationing in tropical or subtropical climates or high altitudes, swimming or spraying your skin with water, tanning under artificial light sources, and mixing outdoor recreation with alcohol. Additionally, having a certain autoimmune disease or medical condition that weakens the immune system; undergoing an organ transplant; having light skin, blue eyes, and red or blond hair; or taking photosensitizing medicines may potentiate a person’s risk for sunburn.1-3,15,17 Photosensitizing medications that increase a patient’s risk for sunburn are numerous and include nonsteroidal anti-inflammatory drugs; antimalarials; various antibiotics, such as sulfonamides, quinolones, and tetracyclines; antihypertensives; antidepressants; and diuretics.1,15
Sunscreen Regulation
The FDA regulates sunscreen as a drug product under the Food, Drug and Cosmetic Act, and its work on the safety and efficacy of sunscreen dates back to 1972.13 In 2019, the FDA proposed new rules for regulating sunscreen products, detailing labeling requirements, considerations for active ingredients, sunscreen dosage forms, and safety information. Until 2019, active ingredients in sunscreen were thought to be generally recognized as safe and effective (GRASE) without required testing. The FDA proposal separates the active ingredients into multiple GRASE categories, outlined in TABLE 1, noting that only GRASE I ingredients do not require further testing to prove safety. GRASE Category I ingredients, which include zinc oxide and titanium dioxide, are considered physical sunscreen barriers and are not absorbed by the skin to any significant degree.13 They act by preventing absorption of UVR from the sun, thereby protecting the skin. GRASE Category II and III ingredients are considered chemical sunscreen agents and work by absorbing UVR from the sun.14
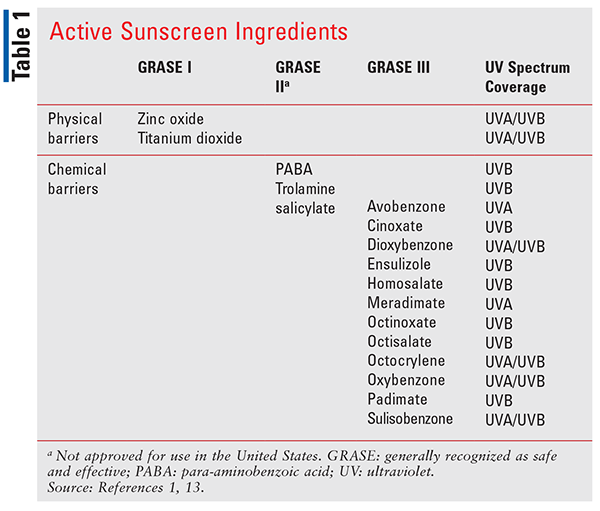
The FDA proposal concludes that risks continue to outweigh the benefits for GRASE II ingredients, which are currently not used in sunscreen products in the U.S.13 The need for further investigation of the GRASE Category III agents was substantiated in a 2019 FDA report, published in the Journal of the American Medical Association. This report indicated that GRASE Category III ingredients, including products such as oxybenzone and padimate O, may be systemically absorbed at higher concentrations than originally believed, and these ingredients require further studies to make a final conclusion about their safety.18
Sunburn prevention begins with an understanding of sun protection factor (SPF) and UVR. SPF is a measure of the amount of UVR required to cause a sunburn while a person is wearing sunscreen.13,14 The FDA proposal also outlines that sunscreens with SPF 15+ and higher must also be broad spectrum, offering protections from both UVA and UVB rays, and that the max SPF labeling for sunscreen would be raised from 50+ to 60+ based on the fact that there is a significant enough difference between the action of sunscreen at these two levels to substantiate the change. Additionally, the proposal contains specific requirements for labeling, including a requirement to list all active ingredients alphabetically, use the word “sunscreen,” and indicate the dosage form, SPF, broad spectrum, and water resistance on the label.13
Sunscreens that do not carry both a minimum SPF 15+ and broad-spectrum coverage will be required to carry the warning that “spending time in the sun increases your risk of skin cancer and early skin aging” and that “this product has been shown only to help prevent sunburn, not skin cancer or early skin aging.”13 The proposal allows for dosage forms of oil, lotion, cream, gel, butter, paste, ointment, stick, and spray to continue manufacturing without the need for new drug applications.13 While the proposal was slated to be formalized in late 2019, it will likely be late 2021 before the proposal is formalized due to delays caused by COVID-19.
Prevention Tips
While the FDA indicates that SPF 15+ is sufficient for skin cancer protection, the American Academy of Dermatology suggests a minimum SPF of 30+ for skin cancer protection. Both agree, though, that broad spectrum coverage is a requirement.13,14,19 In addition to sunscreen and its proper use, sunburn prevention includes nonpharmacologic measures to ensure the best skin protection.
Sunscreen Considerations
• Select appropriate sunscreen for your skin type and activity.
• Apply sunscreen every day to all exposed areas of the body. Most adults require about 1 ounce of sunscreen, or the equivalent of a full shot glass, to ensure all exposed skin is covered.
• Pay careful attention to applying sunscreen to the tops of feet, scalp, tops of ears, and lips.
• Keep sunscreen out of direct sunlight, and the expiration date should be checked with each application.
• Apply sunscreen 15 minutes before going outside and reapply at least every 2 hours, regardless of SPF.
• Reapply water-resistant sunscreen according to package directions, at least every 40 to 80 minutes while in contact with water, as no sunscreen product is completely waterproof.
• Consider sunscreen dosage forms when selecting a product. Creams may be best for dry skin and the face, while sticks may be best for areas around the eyes, and gels may be best for hairy areas.
• Caution patients that sprays should not be used around heat, open flames, or smoking, as they can be flammable. Sprays should not be inhaled and should be rubbed in with the hand after spraying on the body to ensure best coverage.13,14,19,20
Nonpharmacologic Sunburn Prevention
• Minimize sun exposure between 10 AM and 2 PM when the sun’s rays are the strongest.
• Wear protective clothing, including long sleeves, pants, a hat with a wide brim, and sunglasses.
• Stay in shaded areas when you are able.
• Do not use tanning beds.
• Use caution in snow, high altitudes, and water, as these areas reflect the sun’s rays more and may increase your risk of sunburn.
• Minimize sun exposure for children younger than age 6 months, as it is not recommended to use sunscreen in this age group. Consult a pediatrician for guidance in children younger than age 6 months who cannot be kept out of sun.13,14,19,20
Complications and Treatment
General sunburn complications include pain, swelling, redness, warm or pruritic skin, and dehydration, as well as accelerated skin aging.12,16 In contrast, severe sunburn leads to blisters and massive fluid loss with electrolyte imbalances.3,16 When repeated overexposure to UVR occurs, there is a direct relationship to an increase in skin cancer, with five or more sunburns more than doubling your risk of developing melanoma.3,12,21 Although melanoma is the deadliest form of skin cancer resulting from repeated UVR exposure, basal cell carcinoma and squamous cell carcinoma can also occur.12
While the majority of sunburns will heal on their own without further intervention, there are a series of steps that can be taken to treat sunburn.3 First, get out of the sun and avoid the sun to prevent further damage. Next, cool your skin with a short cool shower or bath, or indirectly applied cold compress. This will help relieve pain and discomfort. Add colloidal oatmeal to a bath to provide additional comfort but be sure to avoid using any harsh soaps. Use a moisturizer with aloe vera or soy to soothe the skin for several days. Additionally, applying a moisturizer when the skin is damp provides enhanced hydration to the damaged skin. OTC hydrocortisone (HC) cream 1% can be applied to areas that are particularly uncomfortable; however, do not use HC cream on children unless instructed by the pediatrician. Petrolatum and other oil-based ointments, as well as local anesthetic “-caine” products, should be avoided in all patients as they can worsen the burn and/or increase irritation. Take anti-inflammatory medications such as ibuprofen, naproxen, or aspirin to reduce pain, edema, and inflammation.3,12,16
Aspirin should not be given to children under age 19 years during episodes of fever due to risk of Reye’s syndrome.22 Alternatively, acetaminophen can be used, but only to treat pain.23 Also, increase water intake to avoid dehydration, as sunburn draws fluid to the skin’s surface and away from the rest of the body.3,12,16 Electrolyte supplementation may also be beneficial to restore hydration balance. Rehydrate children using water or juice; however, if the child is not urinating regularly, treat this as an emergency and call the doctor.12 If blisters occur, do not pop them. Blisters form as a protective measure from infection and to aid in the healing process of your skin.16 If blisters burst or pop, clean the area with water, and apply a topical antibiotic along with loose, sterile gauze to protect the burn. As the burn heals, pruritus can occur; adding an antihistamine such as diphenhydramine to treatment would be appropriate.23
Not all sunburns are self-treatable. If the sunburn is severe, it may require a visit to the doctor or hospital. In addition, medical attention should be sought in any of these situations: severe sunburn covering more than 15% of the body; dehydration; fever >101°F; extreme pain persisting for over 48 hours; children older than age 1 year experiencing severe pain, blistering, or lethargy.12,24 Treat sunburn of any child younger than age 1 year as a medical emergency and seek medical treatment immediately.12
Pharmacist’s Role
Pharmacists play a key role in educating patients on the short- and long-term effects of sun exposure, mitigating exposure, and treating overexposure. Counseling patients on appropriate selection and use of sunscreen products could affect sunburn incidence. However, sunscreen is not the only safeguard mechanism against harmful UVR and the risks associated with sunburn. Patients should be advised of additional preventative measures, such as wearing sunshield clothing and limiting exposure. Inform patients how to proceed when sunburn does occur, with emphasis on first getting out of the sun and cooling down the skin; further assessment will dictate which steps to take next. Capitalize on the opportunity to discuss the risks associated with sunburn when discussing treatment and prevention with patients. With this knowledge, they will understand the dangers associated with UVR and can make informed decisions regarding sunburn prevention and treatment.
Conclusion
Sunburn, although generally a minor condition, is both treatable and preventable. Strategies exist to reduce the risk of long-term complications. Treatment options vary widely, and pharmacist knowledge and expertise are key to patient education and overall positive patient outcomes.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. Crosby KM, O’Neal KS. Prevention of sun-induced skin disorders. In: Krinsky DL, ed. Handbook of Nonprescription Drugs: An Interactive Approach to Self-Care. 19th ed. Washington, DC: American Pharmacists Association; 2018:743-756.2. Mayo Clinic. Sunburn-symptoms and causes. www.mayoclinic.org/diseases-conditions/sunburn/symptoms-causes/syc-20355922. Accessed May 21, 2021.
3. Guerra KC, Urban K, Crane JS. Sunburn. NCBI Bookshelf website. www.ncbinlm.nih.gov/books/NBK534837. Accessed April 23, 2021.4. United States Department of Health and Human Services. Skin cancer: quick facts from the Surgeon General. United States Department of Health and Human Services. www.hhs.gov/surgeongeneral/reports-and-publications/skin-cancer/fact-sheet/index.html. Accessed April 23, 2021.
5. Stern RS. Prevalence of a history of skin cancer in 2007: results of an incidence-based model. Arch Dermatol. 2010;146:279-282.6. CDC. Sunburn and sun protective behaviors among adults aged 18-29 years—United States, 2000-2010. MMWR Morb Mortal Wkly Rep. 2012;61(18):317-322.
7. CDC. Sunburn prevalence among adults—United States, 1999, 2003, and 2004. MMWR Morb Mortal Wkly Rep. 2007;56(21):524-528.8. National Cancer Institute. Cancer Trends Progress Report. Sunburn, https://progressreport.cancer.gov/prevention/sunburn. Accessed April 23, 2021.
9. Ambizas EM, Maniara B. Avoiding sunburn: all about sunscreen. US Pharm. 2016;41(7):26-29.10. Narayanan D, Saladi RN, Fox JL. Ultraviolet radiation and skin cancer. Int J Dermatol. 2010;49:978-986.
11. Balk SJ; Council on Environmental Health; Section on Dermatology. Ultraviolet radiation: a hazard to children and adolescents. Pediatrics. 2011;127(3):e791-e817.12. Skin Cancer Foundation. Sunburn and your skin. www.skincancer.org/risk-factors/sunburn/. Accessed April 23, 2021.
13. Federal Register. Sunscreen drug products for over-the-counter human use. Federal Register website. www.federalregister.gov/documents/2019/02/26/2019-03019/sunscreen-drug-products-for-over-the-counter-human-use. Accessed May 17, 2021.14. American Academy of Dermatology Association. Sunscreen FAQs. www.aad.org/public/everyday-care/sun-protection/sunscreen-patients/sunscreen-faqs. Accessed May 17, 2021.
15. Young AR, Tewari A. Sunburn: symptoms and causes. In: UpToDate. Corona R, Ed. UpToDate: Waltham, MA. Accessed April 23, 2021.16. American Academy of Dermatology Association. How to treat sunburn. www.aad.org/public/everyday-care/injured-skin/burns/treat-sunburn. Accessed April 23, 2021.
17. American Cancer Society. Skin cancer prevention and early detection. www.cancer.org/content/dam/CRC/PDF/Public/6930.00.pdf. Accessed April 23, 2021.18. Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321(21):2082-2091.
19. American Academy of Dermatology Association. How to apply sunscreen. www.aad.org/public/diseases/skin-cancer/prevent/sunscreen-apply. Accessed May 17, 2021.20. FDA. Sunscreen: how to help protect your skin from the sun. www.fda.gov/drugs/understanding-over-counter-medicines/sunscreen-how-help-protect-your-skin-sun. Accessed May 17, 2021.
21. Monseau AJ, Reed ZM, Langley KJ, Onks C. Sunburn, thermal, and chemical injuries to the skin. Prim Care. 2015;42:591-605.22. Beutler A, Chesnut GT, Mattingly JC, Jamieson B. Aspirin use in children for fever or viral syndromes. Am Fam Physician. 2009;80(12):1472-1474.
23. Mayo Clinic. Burns: first aid. www.mayoclinic.org/first-aid/first-aid-burns/basics/art-20056649. Accessed May 21, 2021.24. CDC. NIOSH fast facts: protecting yourself from sun exposure. www.cdc.gov/niosh/docs/2010-116/default.html. Accessed April 23, 2021.
To comment on this article, contact rdavidson@uspharmacist.com.





