US Pharm. 2011;36(11):39-44.
The FDA recently released a new safety warning regarding the use of antipsychotics (APs) during pregnancy due to potential negative effects on newborns.1 This warning has sparked the broader question of the overall safety of prescribing APs at any time during pregnancy.2 This question becomes a key issue for women with psychiatric disorders and for the prescribers treating them, since the peak age of onset of such disorders occurs during the childbearing years from 20 to 40.3 It is estimated that annually more than 500,000 pregnancies in the United States occur in women with a pre-existing psychiatric illness or one that emerges during the pregnancy. Of that population of women, approximately one-third are exposed to psychotropic medication at some point during their pregnancy.4 All pharmacists, whether practicing within institutions or the community, are impacted by the FDA required labeling change and must be familiar with the risks and benefits of medications used to treat mental illness in women of childbearing age.
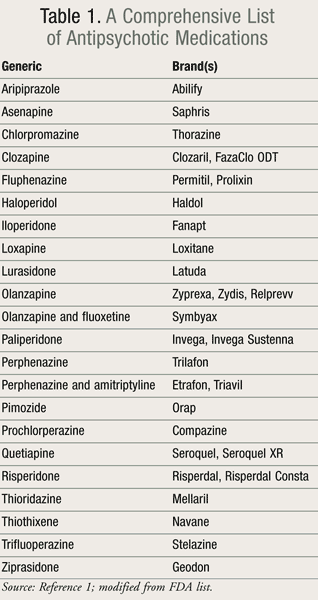
Studies regarding the safety of APs during pregnancy are not readily available, as the study of medication use during pregnancy is limited due to the ethical complications of introducing a new drug to a woman during pregnancy. However, research protocols may include enrollment of a woman into a study upon learning of pregnancy if the woman was receiving the drug at the time of conception. Even with the data available from these studies, little is still known about the ultimate risk to the unborn when the mother is on psychiatric medications. A list of APs is provided in TABLE 1.
The Newest Safety Announcement
In February 2011, the FDA informed health care professionals that the labeling for all APs will be updated to include an enhanced pregnancy section that provides more detailed information for this entire class of medications.1 The enhanced detail focuses on the potential risk in newborns of abnormal movements, known as extrapyramidal symptoms (EPS), which are seen commonly in adults on APs.5 The FDA warns that these abnormal movements may also be a withdrawal syndrome experienced when the in utero antipsychotic exposure is abruptly discontinued at birth, and they are associated with third trimester exposure.1
The FDA’s Adverse Event Reporting System (AERS) documented 69 cases of neonatal EPS or withdrawal across all antipsychotic drugs through October 28, 2008.1 The symptoms reported in these cases included agitation, hypertonia, hyptonia, tremor, somnolence, respiratory distress, and feeding disorder. It was not possible to ascertain whether these reports were related to altered serum concentrations (either toxicity or withdrawal), as blood results were not provided with the reports of adverse events. Further adding to the ambiguity is that the time of onset as well as the severity of symptoms was highly variable, ranging from birth to 1 month after delivery. Some newborns required intensive care and prolonged hospitalization while others recovered a few hours after birth. The confounding effect of concomitant medications could not be discounted and was a possible alternative cause of the withdrawal syndromes reported. The other medication categories seen in the case reports included antidepressants, benzodiazepines, and opioids. There were, however, cases of this syndrome where APs were the only medication exposure during pregnancy.
What Do We Know About the Use of APs During Pregnancy?
Expanded Use of APs
We have seen an increase in the prescribing of antipsychotic medications in the U.S. through new FDA-approved uses as well as increased unapproved FDA use (off-label use). It has been estimated that antipsychotic use for indications without FDA approval increased from 4.4 million visits in 1995 to 9.0 million in 2008.6 Atypical APs are now routinely being used as adjunctive treatment in major depression, in agitation seen with concurrent comorbid disorders, and to decrease sleep latency in patients with insomnia and other sleep disorders. While prescribing medication for off-label indications is becoming a more common practice and does not necessarily reflect inappropriate prescribing, we must recognize that this increased use will result in additional exposure of these agents to pregnant women.

Safety Ratings Can Be Misleading
In recent years, the FDA has worked to develop and include more comprehensive information in drug labels about the drug’s effects during pregnancy as emerging safety information and studies have been released. In 2008, the FDA proposed major revisions to prescription drug labeling concerning drug use in pregnancy and while breastfeeding. These proposed revisions required more complete information about the use of drug and biological products in women of childbearing age and in pregnant and lactating women.7
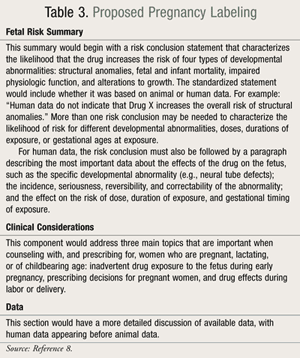
The FDA proposed that both the pregnancy and lactation subsections of labeling include a summary of risk, clinical considerations to support patient care decisions, and educational counseling. The proposed regulations would eliminate the current pregnancy categories A, B, C, D, and X (TABLE 2) due to limitations in their ability to accurately and consistently convey risks and benefits. The proposed subsections would also include a data section with more detailed information. Information about the use of medicines during labor and delivery would be moved to the “Pregnancy” subsection (TABLE 3).8
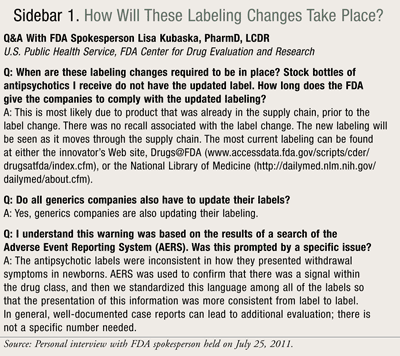
Since February 2011, the Final Rule is still in the writing and clearance process. This process requires identification and consideration of all issues brought forth in the public comments submitted during the 90-day window for comment. Once the Pregnancy and Lactation Labeling Rule is published, the FDA will provide the draft Guidance for Industry and information for health care practitioners, including pharmacists, about these important changes (SIDEBAR 1).7
What Are the Major Risks of Medication Use During Pregnancy?
When considering medication safety in pregnancy, there are three main negative outcomes of fetal exposure. The first is structural teratogenesis, which is more commonly known as a birth defect or congenital malformation. These events occur when a fetus is exposed to certain medications during the development of the major organs and physical structures at 2 to 8 weeks postfertilization. The second kind of effect resulting from medication exposure during pregnancy is behavioral teratogenesis. Although medications can cause these behavioral or neuropsychiatric symptoms, it is extremely difficult to link them to antipsychotic use. This is due to the confounding variable of maternal mental illness, which can independently cause these symptoms. Finally, medication can affect a pregnancy by causing perinatal syndromes. Perinatal syndromes are a cluster of unusual symptoms displayed by the baby after birth as a result of antipsychotic medication administration near the time of delivery. These symptoms are often a result of drug intoxication in the infant, but can also be suggestive of drug withdrawal. Examples of these behaviors include motor restlessness, tremor, feeding difficulties, and hypertonicity.9
The 2011 FDA warning for antipsychotic use and EPS in newborns applied to perinatal syndromes.1 Though this safety alert was specific to third-trimester exposure, health care professionals must consider the safety ratings of all female patients who are of childbearing age. Prescribers should continually confirm safety ratings prior to prescribing during pregnancy, as pregnancy categories can change with emerging safety information and the reporting of postmarketing adverse events.
What Are the Pharmacodynamic and Pharmacokinetic Issues During Pregnancy?
All psychiatric medications studied to date cross the placenta, are present in the amniotic fluid, and can enter human breast milk.4 Knowledge of the gestational age of the fetus is key when making decisions, as the main risk of teratogenesis occurs during the first trimester, with the highest risk during weeks 3 through 8.4
Most pregnancy studies are either prospective cohort studies or retrospective database studies because of the ethical complication of investigational drug use in pregnant females. However, in 2005, a prospective comparative study was published that looked at whether exposure to atypical APs in pregnancy increased the rate of major malformations in newborns. This study used the Motherisk Program, a telephone-based counseling service that provides pregnant women and health care professionals with evidence-based information on safety and risks of fetal exposure to prescription drugs, OTC medications, chemicals, and infectious agents.3 Women who called the hotline to ask about an antipsychotic medication that they took during pregnancy were enrolled in the study after informed consent was obtained. Results from the study provided data on 151 women with exposure to atypical APs during the first trimester of pregnancy; these results were not found to be statistically significant. The authors concluded that atypical APs did not appear to carry an increased risk of major malformations when used in pregnancy. Differences in pregnancy outcomes that were found to be statistically significant included rate of low birth weight (10% in exposure group vs. 2% in control group; P = .05) and rate of therapeutic abortions (9.9% in exposure group vs. 1.3% in control group; P = .003).3
If Medication Is Warranted, What Are the Best Strategies?
A single medication at a higher dose is favored over multiple medications for the treatment of psychiatric illness during pregnancy; however, using the lowest effective dose is the safest prescribing plan. Changing medication during pregnancy increases the exposure to the fetus, so the choice of agent should be based on a history of efficacy for the patient.4 When selecting agents for women of childbearing years, prescribing physicians should select medications with the fewest metabolites, highest protein binding, and fewest drug interactions. Pregnancy results in many pharmacokinetic changes—greater volume of distribution, additional fat stores, and lower protein binding. Medication metabolism can also vary substantially for pregnant women, and proactive dosage modifications may be warranted.10
Prescribing physicians should be fully aware of the risks of the drugs prescribed during pregnancy and should discuss therapeutic goals with the patient. Just as prescribers use blood pressure (BP) medication to reduce the numerical value of BP, clinicians must also consider psychiatric rating scales and diagnostic scores to document the value of the prescribed therapy. If the therapy does not achieve the intended goals, the medication regimen should be re-evaluated to ensure that the benefit truly is greater than the risk.
How Will Future Safety Ratings Be Updated?
Adverse reactions and side effects of drug therapy should be reported. These adverse effects should be reported not only to the prescribing physician, but also to the FDA through the MedWatch system. Recent labeling changes to the selective serotonin reuptake inhibitor paroxetine (Paxil) were a result of reports of cardiac malformations and resulted in changes to the pregnancy category, which better guides the avoidance of this drug during pregnancy.11 The FDA uses data from various sources in its decisions to change labeling, including published studies in the medical literature about the use of prescription drugs during pregnancy and breastfeeding. However, one of the best sources of information is through pregnancy exposure registries that collect information on the effects of already approved drugs prescribed to pregnant women. While not a mandatory requirement, the FDA encourages drug companies to maintain pregnancy registries. Pharmacists, physicians, and nurses can encourage patients to learn more about participating in pregnancy exposure registries by visiting www.womenshealth.gov.

Conclusion
The new model of mental health care delivery is moving towards brief institutional hospitalizations with maintenance and recovery in the community. Integration back into the community and family support are key to establishing long-term control over and recovery from psychiatric symptoms. This increased community integration also lends itself to patients seeking normalized human experiences such as careers, relationships, and family planning. When drug therapy is indicated, the prescriber should be familiar with the risks, and the health care team must educate the patient in order to ensure that the decision made is one that is shared. Nondrug therapy should always be considered as a possible alternative solution where such treatment is appropriately indicated. In general, APs should not be taken by pregnant women unless the benefits outweigh the risks and, in those cases, should be taken only under the supervision of a physician who can manage any potential adverse effects in the fetus (TABLE 4).
REFERENCES
1. FDA Drug Safety Communication: antipsychotic drug labels updated on use during pregnancy and risk of abnormal muscle movements and withdrawal symptoms in newborns. February 22, 2011. www.fda.gov/Drugs/DrugSafety/
2. Pelegrin G, Rouzeau V. Drug therapy during pregnancy: an update on FDA labeling for antipsychotics. Pharmacy Times. June 17, 2011. https://secure.pharmacytimes.
3. McKenna K, Koren G, Tetelbaum M, et al. Pregnancy outcome of women using atypical antipsychotic drugs: a prospective comparative study. J Clin Psychiatry. 2005;66:444-449.
4. ACOG Committee on Practice Bulletins–Obstetrics. ACOG Practice Bulletin: clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001-1020.
5. Stahl SM. Antipsychotic agents. Stahl’s Essential Psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2008:332-341.
6. Alexander GC, Gallagher SA, Mascola A, et al. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Pharmacoepidemiol Drug Saf. 2011;20:177-184.
7. Pregnancy and lactation labeling. FDA. Updated February 11, 2011. www.fda.gov/Drugs/
8. Summary of proposed rule on pregnancy and lactation labeling. FDA. November 12, 2009. www.fda.gov/Drugs/
9. Murphy PJ. The fetal circulation. Contin Educ Anesth Crit Care Pain. 2005;5:107-112.
10. Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44:989-1008.
11. FDA advising of risk of birth defects with Paxil. Agency requiring updated product labeling. FDA news release. December 8, 2005. www.fda.gov/NewsEvents/
To comment on this article, contact rdavidson@uspharmacist.com.





