US Pharm. 2021;46(2):HS2-HS11.
ABSTRACT: Lipid-lowering therapy is used to reduce the risk of atherosclerotic cardiovascular disease (ASCVD), with statins being the drugs of choice. Despite the use of statins, the risk of additional cardiovascular events persists. Based on lipid levels and estimates of ASCVD risk, statins are used in combination with other lipid-lowering therapies, such as ezetimibe, proprotein convertase subtilisin/kexin type 9 inhibitors, and eicosapentaenoic acid–only omega-3 fatty acids. Overall, combinations of fenofibrate or niacin with a statin have failed to reduce ASCVD risk. Potentially effective drugs with novel lipid-lowering mechanisms are currently being investigated in clinical trials for use in patients with ASCVD or familial hypercholesterolemia.
Cardiovascular disease (CVD) remains the most common cause of morbidity and mortality in the United States.1 In the 1950s, it was estimated that one in three men in the U.S. developed coronary heart disease before age 60 years.2 At that time, there was no known treatment capable of prolonging life in patients with CVD. In 1959, the Framingham Study identified increased blood cholesterol as a risk factor for premature heart disease.3 Based on these findings, a number of studies were initiated to evaluate whether lipid-lowering therapy could reduce the morbidity and mortality associated with atherosclerotic CVD (ASCVD).
Studies performed prior to the widespread use of statins found marginal CV benefit with the use of niacin, cholestyramine, and fibric acid derivatives.4 These trials were also conducted before aspirin, beta-blockers, renin-angiotensin-aldosterone system modulators, and percutaneous coronary intervention were routinely used. Therefore, it is difficult to gauge the impact of these older nonstatin drugs on morbidity and mortality in the current era of comprehensive ASVCD risk reduction.
Statins
Statins were first approved for use in the U.S. in the late 1980s to reduce LDL cholesterol (LDL-C) and total cholesterol (TC). Statins inhibit hydroxymethyl glutaryl coenzyme A reductase, an enzyme responsible for the synthesis of cholesterol in hepatocytes.5 Dozens of outcomes trials have demonstrated that statins have a relatively consistent benefit in reducing ASCVD risk in primary and secondary prevention.6 Statins that lower LDL-C to a greater extent (high-intensity; LDL-C reduction of 50% or more) reduce CV events (CVEs) more than statins with a lesser LDL-C–lowering capability (moderate-intensity and low-intensity; LDL-C reduction of 30%-50% and <30%, respectively).7 Statin trials have demonstrated a linear relationship between treated LDL-C levels and clinical outcomes, with lower LDL-C levels resulting in fewer adverse CVEs. These data have led to the adoption of aggressive on-treatment LDL-C targets supported by expert consensus guidelines.7 The guidelines also recommend the use of high-intensity statin therapy in patients with the greatest CV risk, and only two agents are defined as high-intensity statins (TABLE 1).
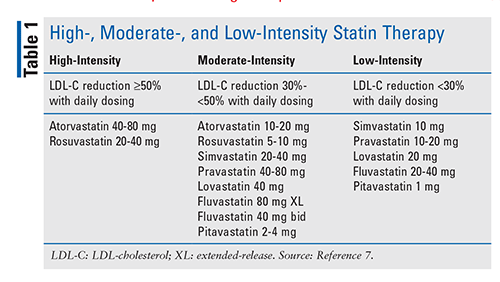
Adherence to statin therapy is poor; approximately 50% of patients stop therapy during the first year of treatment.8 Poor adherence is a multifactorial problem, as only a small portion of patients have statin intolerance. Clinical trials and real-world data report statin-discontinuation rates of 5% to 15% for adverse reactions.9 Some patients may tolerate a low- or moderate-intensity statin but not a high-intensity statin. Other patients may tolerate a specific statin but not other statins.
In patients not achieving desired LDL-C levels, other lipid-lowering drugs may be added to statins in order to further reduce ASCVD risk.4 Results of combination-drug trials have been inconsistent (TABLE 2). The addition of niacin or fenofibrate to a statin failed to produce a significant reduction in CVEs. In a subgroup of patients with atherogenic dyslipidemia (low HDL cholesterol [HDL-C], high triglycerides [TG], and diabetes mellitus [DM]), fenofibrate plus a statin produced a trend toward reducing ASCVD events; however, the overall benefit of fenofibrate plus a statin was no better than a statin alone.10 These results led the FDA to remove fixed-dose combinations of statins with either fenofibrate or niacin from the U.S. market. Bile-acid sequestrants may be added to statin therapy to further reduce LDL-C; however, no major trials evaluating the impact on CVEs have been performed.
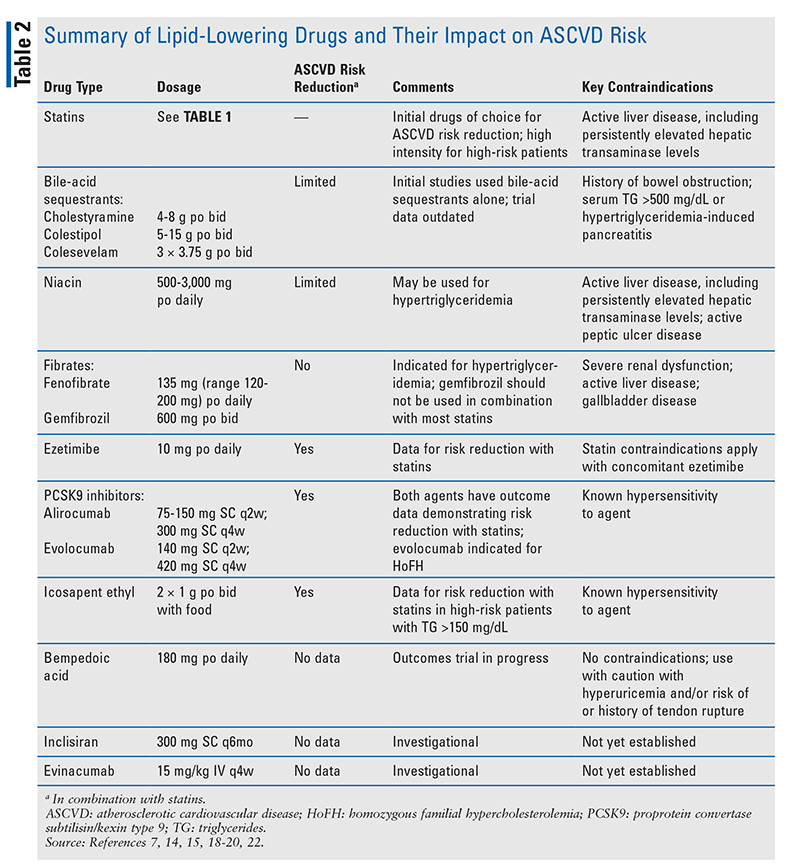
Ezetimibe
Ezetimibe is a small-molecule nonstatin drug that inhibits the cholesterol transporter Niemann-Pick C1-Like 1, which is responsible for cholesterol absorption in the intestine.11 Administered at a fixed dosage of 10 mg daily, ezetimibe reduces LDL-C by 20% to 25% when used alone or in combination with a statin. Ezetimibe absorption is not affected by food, and the drug does not alter fat-soluble vitamin absorption. Dose adjustment is not required in renal dysfunction, but the drug is not recommended in patients with moderate-to-severe hepatic dysfunction. The frequency of adverse reactions leading to drug discontinuation is similar to that for placebo.
In a study of patients recently hospitalized for acute coronary syndrome (ACS), ezetimibe plus simvastatin versus simvastatin alone produced a significant reduction in ASCVD risk.12 Despite the statistical significance, the magnitude of the benefit of combination therapy was small (absolute risk reduction [RR], 2%) considering the large sample size (N = 18,144) and long duration of treatment (median 6 years). Additionally, 42% of patients withdrew from the study prior to completion. The greatest benefit from the combination of ezetimibe and simvastatin was observed in patients aged 75 years and older. The absolute reduction in the primary composite outcome was 0.9% in patients younger than age 65 years, 0.8% in those aged 65 to 74 years, and 8.7% in those aged 75 years and older. In a study conducted in Japan, 3,796 patients aged 75 years and older without a history of coronary disease were randomized to ezetimibe 10 mg daily or usual care.13 After 4-year follow-up, ezetimibe significantly reduced the incidence of cardiac events. Results of this study were limited by the lack of placebo control, slow trial enrollment, premature trial closure with about two-thirds of intended study participants enrolled, and relatively low event rates. However, these results add further support for the use of ezetimibe, a well-tolerated nonstatin drug, in the reduction of CVEs in elderly patients.
PCSK9 Inhibitors
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is an enzyme responsible for degradation of LDL-C receptors in hepatocytes.11 LDL receptors are responsible for removing LDL-C from the blood. Greater PCSK9 activity leads to fewer LDL receptors and higher plasma LDL-C levels. The first drugs to target PCSK9 were the monoclonal antibodies (Mabs) alirocumab and evolocumab, which bind to and inhibit PCSK9 (PCSK9 inhibitors). These drugs have similar therapeutic profiles, producing a 45% to 60% reduction in LDL-C when used alone or in combination with statins.4 Both drugs are given SC at 2- or 4-week intervals. The most common adverse effect of these drugs is injection-site reactions. Since both alirocumab and evolocumab are fully human Mabs, immunologic responses, including allergic reactions and the development of antidrug neutralizing antibodies, are rare.
Both PCSK9 inhibitors have been evaluated in combination with statin therapy in morbidity and mortality studies. Evolocumab was studied in 27,564 patients with established CVD and a baseline LDL-C of 70 mg/dL or greater on maximally tolerated statin.14 Evolocumab produced a significant relative RR of 15% for CVEs. This occurred primarily because of reductions in myocardial infarction (MI), stroke, and coronary revascularization; there was not a decrease in CV death. Alirocumab was studied in 18,924 patients with a recent hospital admission for ACS who had a baseline LDL-C of 70 mg/dL or greater on maximally tolerated statin.15 Similar to evolocumab, alirocumab produced a significant relative RR of 15% for CVEs. The benefit of alirocumab was due to a decrease in MI and stroke, but not CV death. The results of these trials support the addition of PCSK9 inhibitors to statins in patients not achieving LDL-C levels below 70 mg/dL, but data in patients not taking a statin are lacking. Cost-effectiveness concerns continue to limit PCSK9 inhibitor therapy. Although manufacturers recently reduced the cost of these drugs by 60% to approximately $5,800 per year, insurance barriers remain, including unaffordable copayments for some patients.16
Icosapent Ethyl
Prescription omega-3 fatty acids (eicosapentaenoic acid [EPA] and/or docosahexaenoic acid [DHA]) are indicated to treat severe hypertriglyceridemia (TG levels of 500 mg/dL or higher).17 A number of trials investigating various formulations of omega-3 fatty acids demonstrated inconsistent effects on ASCVD risk. These results were most likely related to the use of low doses of omega-3 fatty acids, differences in baseline ASCVD risk in study subjects, and possibly the use of products containing both EPA and DHA. However, a prescription EPA-only omega-3 fatty acid (icosapent ethyl) has been found to significantly reduce ASCVD risk in patients with established CVD or DM who had at least one other CV risk factor, elevated TG (>135 mg/dL-499 mg/dL), and an LDL-C level between 41 g/dL and 100 mg/dL on statin therapy.18
The Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT), which enrolled more than 8,000 subjects and lasted nearly 5 years, demonstrated that patients receiving icosapent ethyl (2 g bid with food) were 25% less likely than placebo patients (P <.001) to experience CV death, nonfatal MI, stroke, coronary revascularization, or unstable angina. Safety issues with icosapent ethyl included increased risk of atrial fibrillation (AF; 5.3% vs. 3.9%; P = .003), greater risk of hospitalization for AF (3.1% vs. 2.1%; P = .004), and higher—though nonsignificant—risk of serious bleeding events (2.6% vs. 2.1%; P = .06). Icosapent ethyl’s benefit in reducing ASCVD risk was unexpected given the previously inconsistent effect of these drugs and the premature discontinuation of a similar mortality study using a high-dose combination (EPA and DHA) prescription omega-3 fatty acid. The specific mechanism of icosapent ethyl in CV risk reduction is unknown but most likely results from the use of an EPA-only omega-3 fatty acid. Based on the findings from REDUCE-IT, the FDA recently approved icosapent ethyl for use in patients taking maximally tolerated statin therapy with baseline TG of 150 mg/dL or higher who have established CVD or DM and two or more additional ASCVD risk factors.
Bempedoic Acid
This oral nonstatin agent reduces LDL-C by inhibiting ATP-citrate lyase, an enzyme in the cholesterol biosynthesis pathway, upstream from the site where statins reduce cholesterol synthesis.19 Bempedoic acid, a prodrug, requires coenzyme A activation by very-long-chain acyl-CoA synthetase-1. This activity occurs solely in the liver and not in the muscle, potentially limiting musculoskeletal complaints. Bempedoic acid does not have substantial effects on lipid fractions other than LDL-C. This agent was approved in early 2020 as an adjunct to diet and maximally tolerated statin therapy in the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or established ASCVD who require additional LDL-C–lowering.
Bempedoic acid is available as a single dosage of 180 mg daily and as a fixed-dose combination with ezetimibe 10 mg daily.19 It is metabolized primarily in the liver via glucuronidation. Although less than 5% of the drug is cleared unchanged, few data exist on patients with severe renal disease (creatinine clearance <30 mL/minute), and there are no data on patients with end-stage renal disease or on hemodialysis. Bempedoic acid is a weak inhibitor of the organic anion transport-2 membrane transporter, which is the most likely mechanism for the increases in serum creatinine and uric acid that can occur during therapy. The drug should be used cautiously in patients with gout. Rare instances of tendon rupture have been reported, but the mechanism and risk factors are unknown. Owing to its metabolism by uridine glucuronosyltransferase, bempedoic acid is associated with an approximately twofold increase in blood levels of simvastatin and pravastatin. Maximal dosages of simvastatin and pravastatin with bempedoic acid are 20 mg daily and 40 mg daily, respectively.
Bempedoic acid has been found to decrease LDL-C by approximately 18% in patients with ASCVD or HeFH who are on maximally tolerated statin therapy.19 In patients with statin intolerance, bempedoic acid was found to decrease LDL-C by 21% (17%-25%) when used alone and by 28% (22%-34%) when used in combination with ezetimibe.19 Currently, no outcomes data are available for bempedoic acid, but an outcomes trial is in progress in patients with statin intolerance (only low-intensity statin therapy allowed) who are at very high risk for or have established ASCVD.
Inclisiran
Inclisiran is a small interfering RNA (siRNA) agent that inhibits production of the PCSK9 protein in hepatocytes.20 Inclisiran was previously known as ALN-PCS, an siRNA conjugated with triantennary N-acetylgalactosamine (GalNAc) carbohydrates.21 This compound was relatively unstable and had to be given IV. The addition of another GalNAc carbohydrate to ALN-PCS improved its stability, allowing the compound (ALN-PCSsc) to be administered by SC injection. The addition of the GalNAc carbohydrate was also clinically important, as it has specific affinity for binding to asialoglycoprotein receptors in the liver. This leads to specific uptake and binding of inclisiran in the liver, with no activity in peripheral tissues.20,21
Inclisiran is a double-stranded RNA molecule that, after uptake in the hepatocyte, binds to a multiprotein complex known as RNA-induced silencing complex (RISC).21 This RISC combines with messenger RNA (mRNA) specific to PCSK9, which induces activation of an enzyme that promotes degradation of the mRNA-RISC complex. The mRNA is degraded, but the active siRNA within the RISC remains active, producing a long-lasting silencing of mRNA targeting PCSK9. Because of its unique mechanism of action, inclisiran reduces intracellular PCSK9 synthesis and has a prolonged duration of action.
Based on phase I and II studies, the maintenance dosage of inclisiran (following two initial doses given 2 months apart) appears to be 300 mg SC every 6 months.21 Researchers performed a pooled analysis of recent studies on inclisiran comprising a large number of patients with HeFH or ASCVD who were randomized to inclisiran 300 mg SC every 6 months (n = 1,883) or placebo (n = 1,827) and followed for an average of 18 months.22 Ninety-two percent of patients received statin therapy (74% received a high-intensity statin). The mean difference in LDL-C at 18 months for inclisiran versus placebo was 55%, and the average reduction from day 90 to day 540 was 52% (both, P <.0001). A greater proportion of inclisiran patients achieved the LDL-C targets of less than 100 mg/dL, less than 70 mg/dL, less than 50 mg/dL, and less than 25 mg/dL, and there were also significant reductions (all, P <.001) in TC, non–HDL-C, lipoprotein(a), and apolipoprotein B (apoB). The only adverse effect occurring more frequently with inclisiran use compared with placebo was injection site reactions (5.0% vs. 0.7%). Inclisiran appears to reduce LDL-C levels to an extent similar to that seen with monoclonal PCSK9 inhibitors, but with less-frequent dosing. In December 2019, a New Drug Application for inclisiran was submitted to the FDA for use in patients with established ASCVD and patients with HeFH.
ANGPTL3 Inhibitor
Angiopoietin-like protein 3 (ANGPTL3) is a hepatically secreted protein that inhibits lipoprotein and endothelial lipase, resulting in decreased levels of TG-rich lipoprotein and HDL-C.23 Inhibition of ANGPTL3 reduces LDL-C levels through an unknown mechanism. Evinacumab, an Mab that inhibits ANGPTL3, was studied in 65 patients with homozygous familial hypercholesterolemia (HoFH).22 Patients had to have genetic mutations in LDL receptors, PCSK9, or apoB with a baseline untreated TC greater than 500 mg/dL and a TG level less than 300 mg/dL. In addition, patients had to be older than age 12 years with an LDL-C greater than 70 mg/dL on maximally tolerated lipid-lowering therapy. Patients were randomized 2:1 to evinacumab 15 mg/kg IV or placebo every 4 weeks for 24 weeks. Evinacumab decreased LDL-C by 49% ± 8% at 24 weeks. LDL-C was decreased by 50% or greater in 56% of evinacumab patients compared with 4.5% of placebo patients. Genetic mutations in the LDL receptor did not appear to affect response to evinacumab. The overall rate of adverse reactions was less with evinacumab than with placebo, but more serious adverse reactions occurred with evinacumab (4.5%) than with placebo (0%). Evinacumab’s unique mechanism of action appears to be independent of LDL-receptor function, which may be particularly valuable in patients with HoFH.
Conclusion
Statins remain the agents of choice for the treatment of dyslipidemia in patients who have or are at substantial risk for established coronary heart disease. Despite statin use, the risk of adverse CVEs persists. The use of other types of lipid-lowering drugs (e.g., ezetimibe, PCSK9 inhibitors, icosapent ethyl) in combination with statins has been shown to further reduce CV risk. Lipid-lowering drugs with novel mechanisms of action are being evaluated for their ability to further reduce CV risk.
REFERENCES
1. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics–2019 update: a report from the American Heart Association. Circulation. 2019;139:e56-e528.
2. Dawber TR, Moore FE, Mann GV. Coronary heart disease in the Framingham study. Am J Public Health Nations Health. 1957;47:4-24.
3. Dawber TR, Kannel WB, Revotskie N, et al. Some factors associated with the development of coronary heart disease: six years’ follow-up experience in the Framingham study. Am J Public Health Nations Health. 1959;49:1349-1356.
4. Ling H, Burns TL, Hilleman DE. Novel strategies for managing dyslipidemia: treatment beyond statins. Postgrad Med. 2012;124:43-54.
5. Stancu C, Sima A. Statins: mechanism of action and effects. J Cell Mol Med. 2001;5:378-387.
6. Cholesterol Treatment Trialists’ (CTT) Collaboration; Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670-1681.
7. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
8. Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288:462-467.
9. Newman CB, Tobert JA. Statin intolerance: reconciling clinical trials and clinical experience. JAMA. 2015;313:1011-1012.
10. ACCORD Study Group; Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
11. Soppert J, Lehrke M, Marx N, et al. Lipoproteins and lipids in cardiovascular disease: from mechanistic insights to therapeutic targeting. Adv Drug Deliv Rev. 2020;159:4-33.
12. Kastelein JJ, Akdim F, Stroes ES, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431-1443.
13. Ouchi Y, Sasaki J, Arai H, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (EWTOPIA 75): a randomized, controlled trial. Circulation. 2019;140:992-1003.
14. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
15. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097-2107.
16. Fonarow GC, van Hout B, Villa G, et al. Updated cost-effectiveness analysis of evolocumab in patients with very high-risk atherosclerotic cardiovascular disease. JAMA Cardiol. 2019;4:691-695.
17. Hilleman DE, Wiggins BS, Bottorff MB. Critical differences between dietary supplement and prescription omega-3 fatty acids: a narrative review. Adv Ther. 2020;37:656-670.
18. Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22.
19. Saeed A, Ballantyne CM. Bempedoic acid (ETC-1002): a current review. Cardiol Clin. 2018;36:257-264.
20. Dyrbus K, Gasior M, Penson P, et al. Inclisiran—new hope in the management of lipid disorders? J Clin Lipidol. 2020;14:16-27.
21. Catapano AL, Pirillo A, Norata GD. New pharmacological approaches to target PCSK9. Curr Atheroscler Rep. 2020;22:24.
22. Jia X, Al Rifai M, Liu J, et al. Highlights of studies in cardiovascular disease prevention presented at the 2020 American College of Cardiology annual scientific session. Curr Atheroscler Rep. 2020;22:32.
23. Nurmohamed NS, Dallinga-Thie GM, Stroes ES. Targeting apoC-III and ANGPTL3 in the treatment of hypertriglyceridemia. Expert Rev Cardiovasc Ther. 2020;18:355-361.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






