 Hannah Pope, PharmD, MHA, BCPS, has been a practicing clinical pharmacy specialist for over a decade. Over the last 8 years she has worked at Barnes Jewish Hospital in Missouri alongside a team of physicians that manage patients in the cardiovascular intensive care unit, many presenting with STEMI and high-risk NSTEMI, as well as in the cardiac catheterization laboratory to provide pharmacy support surrounding selection of medications and dosing, operational procedures, formulary changes, protocol development, and drug education for hospital staff.
Hannah Pope, PharmD, MHA, BCPS, has been a practicing clinical pharmacy specialist for over a decade. Over the last 8 years she has worked at Barnes Jewish Hospital in Missouri alongside a team of physicians that manage patients in the cardiovascular intensive care unit, many presenting with STEMI and high-risk NSTEMI, as well as in the cardiac catheterization laboratory to provide pharmacy support surrounding selection of medications and dosing, operational procedures, formulary changes, protocol development, and drug education for hospital staff.
How did the opportunity to add IV KENGREAL® (cangrelor) to formulary present itself?
There was so much excitement surrounding KENGREAL that the day it received FDA approval, I was approached by two interventional cardiologists (ICs) who asked me if I could have it available at the pharmacy the very next day. At that time, because our institution was part of a larger health system with about 10–12 hospitals in its network, formulary approval had to be granted by representatives from all its affiliates. As the clinical pharmacy specialist dedicated to the catheterization lab, my responsibilities include partnering with physicians to facilitate formulary updates and protocol development, so I became the primary champion tasked with making that happen.
What was the unmet need KENGREAL could fill at your hospital?
As a parenteral antiplatelet agent with a favorable pharmacokinetic and pharmacodynamic profile, KENGREAL offered multiple benefits. From a pharmacy standpoint, the rapid onset within 2 minutes1, half-life of ~3–6 minutes1, and offset within 1 hour1 really stood out to me. KENGREAL could be used in a variety of clinical situations such as emergent cases, cases with high angiographic risk, when there is an inability to administer or reliably absorb oral medication, in patients who may need to proceed to surgery, and many others.1 KENGREAL also offers potent platelet inhibition with a pharmacodynamic effect of >98% inhibition of platelet aggregation demonstrated in whole blood impedance aggregometry.2 Furthermore, since KENGREAL is not renally cleared and its metabolism is independent of hepatic function, no dosage adjustments are required in patients with renal or hepatic impairment.1 As a large academic institution, we perform a high volume of PCI procedures, many considered complicated, so there was definitely a role for KENGREAL in our hospital’s antiplatelet armamentarium based on its profile and the types of cases we see.
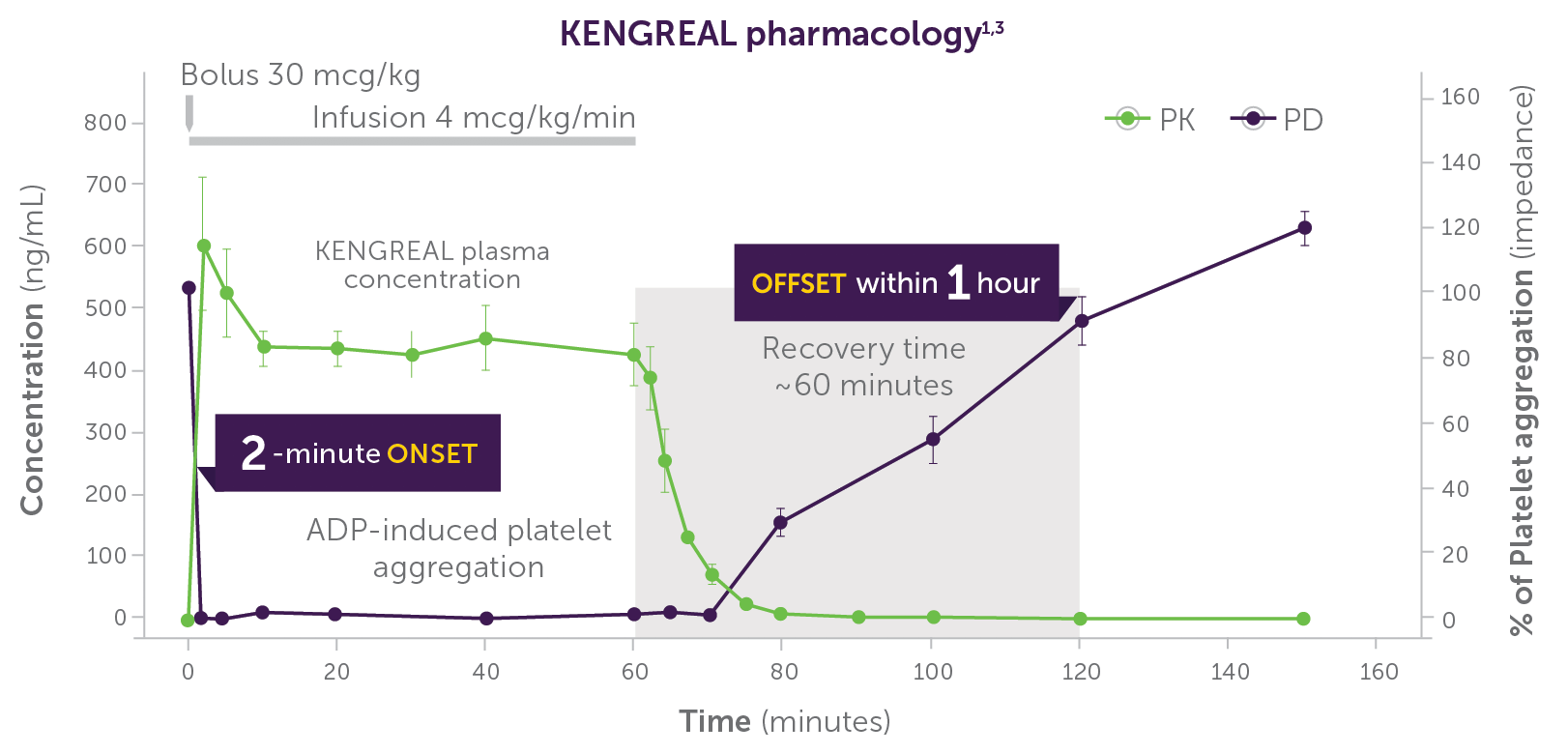
Phase I study in healthy volunteers (n=9); dose: 30 mcg/kg IV bolus + 4 mcg/kg/min IV infusion. KENGREAL blood levels and platelet activity were assessed over 150 minutes by whole blood impedance aggregometry in response to 20 µM of ADP.3
Infusion should be continued for at least 2 hours or the duration of the procedure, whichever is longer.1
Important Safety Information
Drugs that inhibit platelet P2Y12 function, including KENGREAL®, increase the risk of bleeding. In CHAMPION PHOENIX, bleeding events of all severities were more common with KENGREAL® than with clopidogrel. Bleeding complications with KENGREAL® were consistent across a variety of clinically important subgroups. Once KENGREAL® is discontinued, there is no antiplatelet effect after an hour.
Please see Full Important Safety Information.
What were the steps in the process of adding KENGREAL to formulary?
I approached the Pharmacy and Therapeutics (P&T) committee, which is responsible for all matters related to medication use in our network, and requested KENGREAL be added to formulary and available for use as soon as possible in the cardiac catheterization lab. The P&T committee denied the original request and asked to establish and resubmit with prespecified criteria for use. In collaboration with several ICs within the hospital system, we identified target patient populations that present to the cardiac catheterization lab that could benefit from the use of KENGREAL. The initial use criteria were largely derived from the KENGREAL Prescribing Information and AHA/ACC Guidelines regarding use of P2Y12 inhibitors in PCI.*4 We also took into consideration results from CHAMPION PHOENIX, the pivotal trial of KENGREAL vs clopidogrel.5 The criteria defined three subsets of patients in the catheterization lab undergoing PCI at a level specific enough to limit use, but also broad enough to give the treating physician an opportunity to consider KENGREAL for a range of patients within each category.
*KENGREAL is not included in the existing 2014 AHA/ACC Guidelines. KENGREAL was approved by the FDA in 2015.
Once KENGREAL was available on formulary, was there validation required by the hospital system to justify and maintain its addition?
After the P&T committee granted formulary approval, I was tasked with performing a 6-month drug use evaluation (DUE) of every patient who received KENGREAL. It was intended to validate whether the appropriate population was correctly identified and if new criteria should be added; it did not evaluate patient outcomes. The DUE ended up taking 1.5 years, which was encouraging in a way, because it suggested there was no excessive use of KENGREAL and indicated that our pharmacy budget was not significantly impacted by its availability. Ultimately, the vast majority of patients fell within the defined parameters—a successful result. If that were not the case, the P&T committee would likely have asked me to narrow the criteria.
Predefined use criteria for KENGREAL†:
- STEMI: patients in whom glycoprotein IIb/IIIa inhibitor use is not planned; bivalirudin or heparin will be used; patients not loaded with an oral P2Y12 inhibitor prior to PCI.
- NSTEMI/Unstable Angina: patients who were not loaded with an oral P2Y12 inhibitor and planned to go to catheterization lab within 2 hours from presentation for PCI.
- Elective PCI: in high-risk, stable patients with complex anatomy undergoing elective PCI who have not been loaded with an oral P2Y12 inhibitor.
†These criteria are for illustrative purposes only and are more specific than the FDA-approved indication.
Important Safety Information
KENGREAL® (cangrelor) for Injection is contraindicated in patients with significant active bleeding.
Please see Full Important Safety Information
How have the criteria changed since initial approval?
There were no revisions following the first use evaluation, but over time, the criteria have continued to evolve due to identification and validation of additional cases from physicians or as a result of subsequent DUEs that showed other patient types that could benefit from KENGREAL. After the last changes to the use criteria, KENGREAL is well positioned for a variety of PCI cases as defined, and is now a part of the antiplatelet regimen at our hospital.
In addition to any industry-supported education, was it necessary to educate the staff following formulary approval?
Continued staff education is very important, and while I present an annual antiplatelet therapy review, I also wanted to provide focused outreach to physicians, fellows, and nurses following KENGREAL’s availability on formulary. Education was not only targeted to the catheterization lab staff, but it also included other departments. This was because patients receiving KENGREAL may be transferred to other locations in the hospital outside of the catheterization lab, such as the ICU or another floor while the drug is still being administered. With KENGREAL being a newly available antiplatelet agent at our hospital, these informational presentations and the resources provided by the manufacturer were vital to controlling responsible uptake and familiarizing the staff with KENGREAL use. Ensuring safe administration was also an important education component given the need to transition patients to an oral P2Y12 inhibitor to maintain platelet inhibition once the KENGREAL infusion is stopped.
For other clinical pharmacy specialists or institutions looking to add KENGREAL to formulary, what is the best advice you could share? What about for a non-pharmacy specialist who hopes to have it available at their hospital?
Ultimately, it’s most important to not lose sight of your institution’s required formulary process. Some institutions may require an assessment of potential direct cost benefits related to improved outcomes, as well as indirect cost benefits, such as decreased length of stay. At our hospital, gaining formulary approval for KENGREAL required evaluating its clinical benefits5 and pharmacologic profile1, and then identifying appropriate patients and situations for KENGREAL. Establishing use criteria, providing continued education, and performing periodic DUEs helps reassure the P&T committee that the agreed-upon scope of use will be maintained so that KENGREAL may continue to be an important antiplatelet option available to our physicians and for our patients.
Indication
KENGREAL® (cangrelor) for Injection is a P2Y12 platelet inhibitor indicated as an adjunct to percutaneous coronary intervention (PCI) to reduce the risk of periprocedural myocardial infarction (MI), repeat coronary revascularization, and stent thrombosis (ST) in patients who have not been treated with a P2Y12 platelet inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor.
Important Safety Information
KENGREAL® (cangrelor) for Injection is contraindicated in patients with significant active bleeding.
KENGREAL® is contraindicated in patients with known hypersensitivity (e.g., anaphylaxis) to cangrelor or any component of the product.
Drugs that inhibit platelet P2Y12 function, including KENGREAL®, increase the risk of bleeding. In CHAMPION PHOENIX, bleeding events of all severities were more common with KENGREAL® than with clopidogrel. Bleeding complications with KENGREAL® were consistent across a variety of clinically important subgroups. Once KENGREAL® is discontinued, there is no antiplatelet effect after an hour.
The most common adverse reaction is bleeding.
Please see Full Prescribing Information.
KENGREAL® is a registered trademark of Chiesi Farmaceutici S.p.A. ©2021 Chiesi USA, Inc. All rights reserved. 06/21 PP-K-0696 V1.0







