US Pharm. 2006;7:HS-23-HS-28.
Ventricular
arrhythmias are disturbances in cardiac impulse generation or conduction that
occur below the level of the bundle of His, which separates the atrial and
ventricular tissues. These dysrhythmias include premature ventricular
contractions (PVCs), ventricular tachycardias (VTs), ventricular fibrillation
(VF), and sudden cardiac death (SCD). The rhythm disorders can be benign or
lethal and occur more often in patients with structural heart disease.
Ventricular Arrhythmias
Premature
Ventricular Contractions: PVCs are also known as ventricular
premature beats and are characterized as abnormal, wide QRS complexes that
occur prior to normal ventricular depolarization. PVCs are caused by ectopic
ventricular pacemakers and usually last longer than 0.12 seconds.1,2
PVCs may be seen in patients with or without structural heart disease.
Several studies have shown that PVCs are present in about 30% of healthy
people between the ages of 20 and 59 and in approximately 70% to 100% of those
older than 60.3 In addition, according to the Atherosclerosis Risk
in Communities study, PVCs are more common in men, African-Americans, and
those with heart disease.4
In general, ventricular
arrhythmias (including PVCs) are linked to many cardiac and noncardiac causes.
Common cardiac conditions that may predispose patients to these arrhythmias
include ischemic heart disease, heart failure/cardiomyopathy, and valvular
disease. Noncardiac causes of ventricular arrhythmias include stimulants, such
as caffeine and cocaine, electrolyte disturbances, acidosis, and drugs such as
antipsychotics, tricyclic antidepressants, and antiarrhythmics.2
Patients with PVCs may be asymptomatic or may experience palpitations, angina,
hypotension, or worsening heart failure.1
Treatment for PVCs is based on
the presence of structural heart disease and symptoms. In patients with no
evidence of heart disease, PVCs are generally not associated with an increased
risk of mortality.1 The frequency of these palpitations may be
diminished by reducing or eliminating smoking, excessive caffeine consumption,
alcohol intake, and use of drugs such as amphetamines. In addition, any
electrolyte abnormalities, such as hypokalemia and hypomagnesemia, should be
corrected.3 If otherwise healthy patients present with multiple
PVCs and have severe symptoms, beta-blockers are the treatment of choice.
Other options include amiodarone, flecainide, and radiofrequency ablation.
2
The results of the Cardiac
Arrhythmia Suppression Trial (CAST) and CAST II are responsible for
dramatically changing treatment strategies for patients with heart disease who
experience PVCs. CAST's objective was to determine whether antiarrhythmic
drugs would reduce the risk of cardiac arrest and total mortality for post-MI
patients with asymptomatic PVCs. The study was terminated prematurely due to
the higher total mortality and increased rates of death from arrhythmia and
cardiac arrest with flecainide and encainide. CAST II showed that moricizine
was also associated with an increased mortality in the same patient
population. Thus, patients with structural heart disease and asymptomatic PVCs
should not receive drug treatment, and those with symptoms should be treated
with beta-blockers, which have been shown to decrease mortality in post-MI
patients.1 Use of sotalol, which has beta-blocking properties, was
shown to reduce overall mortality post-MI; however, the Survival With Oral
D-Sotalol trial showed that d-sotalol (an isomer with no beta-blocking
properties) increased mortality.5 Amiodarone reduced mortality in
post-MI patients with frequent PVCs in the Canadian Amiodarone Myocardial
Infarction Arrhythmia Trial but had no mortality benefit in post-MI patients
who had left ventricular dysfunction in the European Myocardial Infarct
Amiodarone Trial. Amiodarone is a reasonable second-line therapy for patients
with heart disease experiencing symptomatic PVCs when beta-blockers are
ineffective or not tolerated.5
Nonsustained Ventricular
Tachycardia: VT is defined as three or more PVCs at a rate greater
than 100 bpm and can be further classified as nonsustained VT (NSVT) or
sustained VT depending on the duration of the dysrhythmia. NSVT usually
spontaneously terminates within 30 seconds and is not associated with
hemodynamic instability. The severity of symptoms seen with VT
depends on the duration of the arrhythmia, the ventricular rate, and the
degree of the patient's heart disease. Patients who have NSVT, especially
those without heart disease, are often asymptomatic or have mild symptoms,
such as palpitations, dizziness, and syncope.1
As with PVCs, treatment
options for NSVT depend on the presence of structural heart disease (Table 1
) In addition, treatment is based on the patient's left ventricular function.
Patients without structural heart disease do not require treatment unless they
are symptomatic. Beta-blockers are the preferred therapy in this instance. For
post-MI patients experiencing NSVT, treatment is based on left ventricular
ejection fraction (LVEF). Post-MI patients with LVEF greater than 40% and NSVT
should receive a beta-blocker, regardless of symptoms. Patients with LVEF less
than 40% and NSVT should undergo electrophysiologic (EP) testing to determine
if the dysrhythmia is inducible. An implantable cardioverter defibrillator
(ICD) should be used to treat inducible VT, and a beta-blocker or amiodarone
should be used to treat noninducible VT.1

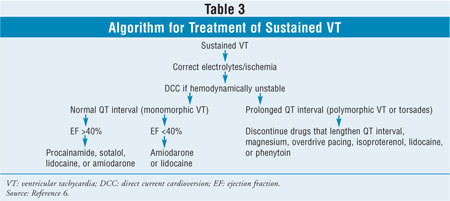
Sustained Ventricular
Tachycardia: Sustained VT can be classified as monomorphic or
polymorphic, depending on the consistency of the QRS complexes on
electrocardiogram. Torsades de pointes is a polymorphic VT that is associated
with a prolonged QTc interval (Table 2). Both types of VT may be caused
by acute MI, severe electrolyte abnormalities, hypoxemia, or drugs.6
Patients with sustained VT are usually symptomatic, and the arrhythmia can
deteriorate into hemodynamic instability with severe hypotension, angina,
and/or syncope. If VT goes untreated, it can deteriorate into VF and possibly
cause death.1 Treatment (Table 3) for any hemodynamically
unstable VT is direct current cardioversion (DCC), which decreases the risk of
VF.5 In addition, all patients with sustained VT should receive
replacement electrolytes, if needed. Drug treatment for sustained monomorphic
VT is based on the patient's left ventricular function. For patients who have
an LVEF greater than 40%, the preferred antiarrhythmic is intravenous (IV)
procainamide, although lidocaine or amiodarone is an acceptable alternative.
7 For patients with left ventricular dysfunction (LVEF <40%), amiodarone
and lidocaine are the preferred agents because neither of these drugs acts as
a negative inotrope. When treating torsades, it is important to discontinue
any drug that may cause a prolonged QTc interval (Table 2). IV
magnesium sulfate is usually considered the drug of choice for treatment of
torsades, regardless of the patient's magnesium level. Since torsades may be
caused or worsened by bradycardia, therapies that increase the heart rate may
be effective for conversion to normal sinus rhythm. These treatments include
insertion of a temporary transvenous pacemaker for overdrive pacing and
isoproterenol. Finally, lidocaine or phenytoin may be effective for
termination of torsades.7

Pulseless Ventricular Tachycardia/Ventricular Fibrillation
VF is defined as the absence of any
organized electrical cardiac activity. As a result of total ventricular chaos,
there is a loss of cardiac output, blood pressure, and pulse.1 If
resuscitation efforts are not made within five to seven minutes, death is
inevitable.8 Indeed, most cases of SCD result from VF or
VT.9 VF and sustained VT without a pulse can be successfully
treated only with DCC, and patients with these dysrhythmias should be managed
according to the current advanced cardiovascular life support (ACLS)
guidelines published by the American Heart Association (AHA; Table 4)
10 The ACLS guidelines for pulseless VT and VF recommend using up to
three shocks with increasing energy (200 joules, 200 to 300 joules, 360
joules) for initial treatment of these arrhythmias. If the dysrhythmia is
persistent or recurrent, the next option is to administer epinephrine 1 mg IV
push every three to five minutes or vasopressin 40 units IV push. The drug
should be followed by one 360-joule shock. If epinephrine or vasopressin with
a subsequent shock is unsuccessful, an additional antiarrhythmic agent should
be given, again followed by DCC. Amiodarone 300-mg IV (or a 5-mg/kg) bolus is
probably the preferred drug for refractory VF, as the results of the
Resuscitation of Refractory Sustained Ventricular Tachyarrhythmias (ARREST)
and the Amiodarone versus Lidocaine in Prehospital Ventricular Fibrillation
Evaluation (ALIVE) studies showed that amiodarone was more effective than
placebo (ARREST) or lidocaine (ALIVE) in survival to hospital admission.
6,11,12 Other antiarrhythmic choices include lidocaine, magnesium, and
procainamide. If a drug is successful in resuscitating the patient, it should
be continued until the patient is stable.6

Sudden Cardiac Death
SCD is defined as
death from a cardiac cause within one hour of onset of clinical symptoms.1
Approximately 80% of patients with SCD have a history of coronary heart
disease.9 As previously mentioned, SCD usually results from VF or
VT. In a study of out-of-hospital arrests, 62% were due to VF, 7% to VT, and
31% to asystole or other arrhythmias. Prevention is the most effective
"treatment" of SCD, as it is the single leading cause of death in the United
States; less than 5% of patients are successfully resuscitated.13
Prevention of SCD from cardiac
causes is divided into two basic strategies: primary prevention and secondary
prevention. The focus of primary prevention is treating patients who are
post-MI or have cardiac disease, in order to prevent a life-threatening
arrhythmia. The goal of secondary prevention is to prevent the recurrence of a
fatal arrhythmia.14 The primary prevention studies Multicenter
Automatic Defibrillator Implantation Trial (MADIT), Multicenter Unsustained
Tachycardia Trial (MUSTT), and MADIT II showed the survival benefit of a
prophylactic ICD in patients with both cardiac disease and left ventricular
dysfunction. With the exception of the EP-guided antiarrhythmic agents used in
MUSTT, the antiarrhythmic drugs in these studies were not generally associated
with a mortality benefit.1,15 Mortality benefit has been shown in
this patient population with several other drugs, including beta-blockers,
aspirin, statins, angiotensin-converting enzyme inhibitors, and spironolactone.
15 Secondary prevention studies, such as the Antiarrhythmics Versus
Implantable Defibrillators, the Canadian Implantable Defibrillator Study, and
Cardiac Arrest Study Hamburg, have shown the survival benefit of ICDs in
patients who have already experienced cardiac arrest. A meta-analysis of these
studies showed a 28% relative reduction in the risk of overall death and a 50%
relative reduction in the risk of death from arrhythmia in patients receiving
an ICD compared to those receiving amiodarone.1 Based on the
results of the secondary prevention trials, ICDs are indicated for first-line
treatment in patients with a history of VT or VF. Amiodarone or sotalol can be
added to the therapy of patients who experience frequent ICD discharges.1
Role of the Pharmacist in
Ventricular Arrhythmias
Pharmacists have an important role
in the treatment of patients with cardiac arrhythmias. Antiarrhythmic
medications are associated with many adverse effects, as well as numerous drug
interactions. Pharmacists must closely monitor patients to ensure that they
are receiving effective therapy with minimal side effects. Patients should
receive extensive education to recognize symptoms associated with toxic
effects of individual antiarrhythmics or a combination of these agents. In
addition, patients should check with their pharmacist or physician before
selecting any OTC medication and should avoid alternative treatment or herbal
supplementation.
Some pharmacists are ACLS
certified and can assist patients who require treatment for life-threatening
arrhythmias. Pharmacists should monitor patients at risk for proarrhythmia for
electrolyte disturbances and any sign or symptom of arrhythmia. Pharmacists in
all practice settings should be familiar with automated external
defibrillators, which are available in many public places. The AHA provides
training to health care workers and family members in using these lifesaving
devices.
Conclusion
Management of cardiac arrhythmias
has changed drastically in the last decade due to the success of procedures
such as radiofrequency ablation and surgery and the development of the ICD.
Antiarrhythmic drug therapy has many limitations, including adverse effects,
drug interactions, and proarrhythmic potential. However, millions of Americans
suffer from arrhythmias, and many patients rely on these drugs to control
symptoms and increase quality of life. It is important for pharmacists to have
an understanding of arrhythmias as well as of prevention and treatment of
these disorders.
References
1. Sanoski C.
Chronic Arrhythmia Management. Pharmacotherapy Self-Assessment Program.
5th Ed. American College of Clinical Pharmacy; 2004:191-227.
2. Hebbar AK, Hueston
WJ. Management of common arrhythmias: part II. Ventricular arrhythmias and
arrhythmias in special populations. Am Fam Physician. 2002;65:2491-2496.
3. Carabello BA,
Ballard WL, Gazes PC. Cardiology Pearls. Philadelphia: Hanley & Belfus,
Inc; 1994:34.
4. Simpson RJ Jr,
Cascio WE, Schreiner PJ, et al. Prevalence of premature ventricular
contractions in a population of African American and white men and women: the
Atherosclerosis Risk in Communities (ARIC) study. Am Heart J.
2002;143:535-540.
5. Chow MSS, White M.
Cardiac arrhythmias. In: Koda-Kimble MA, Young YY, eds. Applied
Therapeutics: The Clinical Use of Drugs. 7th ed. Baltimore: Lippincott
Williams & Wilkins; 2001:18-1–18-36.
6. Bauman JL, Schoen
MD. Arrhythmias. In: DiPiro JT, Talbert LT, et al, eds. Pharmacotherapy: A
Pathophysiologic Approach. 6th ed. New York: McGraw-Hill; 2005:321-356.
7. Cheng JWM. Acute
Management of Arrhythmias. Pharmacotherapy Self-Assessment Program. 5th
ed. American College of Clinical Pharmacy; 2004:41-61.
8. Dresing T.
Tachyarrhythmias. In: Marso SP, Griffin BP, Topol EJ, eds. Manual of
Cardiovascular Medicine. Philadelphia: Lippincott Williams & Wilkins;
2000:249-280.
9. Chow MS. Advanced
cardiac life support controversy: where do antiarrhythmic agents fit in?
Pharmacotherapy. 1997;17(2 pt 2):84S-88S.
10. American Heart
Association in collaboration with the International Liaison Committee on
Resuscitation. Guidelines 2000 for cardiopulmonary resuscitation and emergency
cardiovascular care. Circulation. 2000;102(8):I1-I384.
11. Kudenchuk PJ, Cobb
LA, Copass MK, et al. Amiodarone for resuscitation after out-of-hospital
cardiac arrest due to ventricular fibrillation. N Engl J Med.
1999;341:871-878.
12. Dorian P, Cass D,
Schwartz B, et al. Amiodarone as compared with lidocaine for shock-resistant
ventricular fibrillation. N Engl J Med. 2002;346:884-890.
13. Zhang J. Sudden
cardiac death: implantable cardioverter defibrillators and pharmacologic
treatments. Crit Care Nurs Q. 2003;26:45-49.
14. Huikuri HV,
Castellanos A, Myerburg RJ, et al. Sudden death due to cardiac arrhythmias.
N Engl J Med. 2001;345:1473-1482.
15. Zipes DP.
Epidemiology and mechanisms of sudden cardiac death. Can J Cardiol.
2005;21(suppl A):37A-40A.
To comment on this article, contact
editor@uspharmacist.com.






