US Pharm. 2010;35(3):44-49.
Otitis media (OM) is a common illness affecting both infants and children, often multiple times during the first few years of life. Approximately 16 million office visits and 13 million antibiotic prescriptions during the year 2000 were associated with OM.1 OM has many degrees of severity, including acute OM (AOM), OM with effusion (OME), and chronic suppurative OM (CSOM).
AOM is one of the most frequently occurring childhood diseases, second to upper respiratory infections. It is the leading cause of physician visits, antimicrobial therapy, and pediatric surgery in several countries.2 In the United States, AOM is diagnosed more than 5 million times annually.3 Although AOM can occur in adults, approximately 80% of cases occur in children, with the greatest incidence occurring in those aged 6 to 9 months. By 1 year of age, an estimated 75% of infants will have encountered one episode of AOM, while 17% will have suffered from at least three episodes.2
AOM develops after bacteria invade the middle ear. Factors leading to this invasion include nasopharyngeal colonization, upper respiratory tract infections, and eustachian tube dysfunction.4 After viral respiratory tract infections, ciliary activity in respiratory mucosal cells may be impaired, leading to decreased protection from invading organisms. The eustachian tubes of children aged less than 2 years are shorter than in older children, resulting in shorter distances for bacteria to travel and increasing the probability of infection.2 Additionally, the opening mechanism of the eustachian tubes may be impaired in infants and young children. Furthermore, genetic, infectious, immunologic, and environmental factors can predispose children to ear infections (TABLE 1).5,6
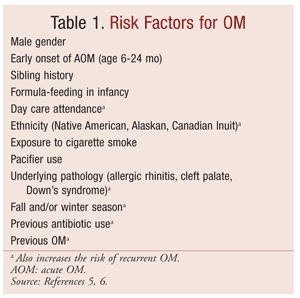
A clinical-practice guideline for the diagnosis and management of AOM was developed by the American Academy of Pediatrics and the American Academy of Family Physicians (AAP/AAFP). According to this guideline, for a diagnosis to be considered “certain,” three specific criteria need to be met: rapid onset, confirmed presence of middle-ear effusion (MEE), and signs and symptoms of middle-ear inflammation. Signs and symptoms of middle-ear inflammation may be indicated by either distinct erythema of the tympanic membrane or distinct otalgia. In infants, pulling of the ear is often indicative of otalgia. Other signs and symptoms pediatric patients may present with, although not specific to AOM, include irritability in infants or toddlers, discharge from the ear, and fever. The presence of MEE is indicated by bulging of the tympanic membrane (the best predictor of AOM), limited or absent mobility of the tympanic membrane, air-fluid level behind the tympanic membrane, or otorrhea.7
AOM can progress to either persistent AOM or recurrent AOM. Persistent AOM occurs when there are persistent features of a middle-ear infection during antibiotic treatment or when a relapse occurs within 1 month of completion of therapy. If a child experiences three or more episodes of AOM within 6 to 18 months, the diagnosis of recurrent AOM should be made.5
OME, or secretory OM, is characterized by fluid in the middle ear without signs or symptoms of infection. It usually occurs when the eustachian tube is blocked and fluid becomes trapped in the middle ear.8 OME may occur spontaneously as part of rhinosinusitis (inflammation of the nasal cavity and sinuses), or it may succeed a bout of AOM.9 Approximately 90% of cases occur in children, with the greatest incidence taking place between 6 months and 4 years of age.10
CSOM, the severest form of OM, is usually a complication of AOM.9,11 A persistent inflammation of the middle-ear cavity or mastoid air cells, CSOM is characterized by a discharge through a perforated tympanic membrane.5 The condition usually is present for more than 2 to 6 weeks.9
Microbiology
Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis are the most common pathogens isolated from AOM, according to studies.5,7 When middle-ear fluid was examined, S pneumoniae, H influenzae, and M catarrhalis were found 25% to 35%, 15% to 30%, and 3% to 20% of the time, respectively.7 Evidence suggests that microbiology may be changing owing to routine vaccination with the heptavalent pneumococcal vaccine; research revealed an increase in H influenzae isolates from 39% to 52% in children aged 7 to 24 months and a decrease in S pneumoniae from 49% to 34% between 1992 and 1998 and between 2000 and 2003.7 Although rare, other pathogens that may be present in AOM include group A streptococcus (GAS), Staphylococcus aureus, anaerobic organisms, Gram-negative bacilli, viruses, Mycoplasma pneumoniae, Chlamydia pneumoniae, and Chlamydia trachomatis (in infants <6 months). S pneumoniae, H influenzae, M catarrhalis, GAS, and S aureus also are responsible for recurrent and persistent OM.7 The bacterial pathogens associated with OME are the same as those seen in AOM. In CSOM, however, Pseudomonas aeruginosa, S aureus, Proteus mirabilis, and Klebsiella species are the most common pathogens isolated, and mixed infections are common. It is important to note that multidrug antibiotic resistance is common with Pseudomonas infections.5,9
Traditional Pharmacologic Therapy
Symptom resolution and reduction of recurrence are the goals of treatment for AOM. Analgesics in addition to oral and topical antimicrobials are the mainstay of treatment. The use of antibacterial agents in children with uncomplicated AOM at the time of diagnosis has raised concerns regarding antibacterial resistance; however, AOM is the most common childhood infection for which antibacterial agents are prescribed in the U.S.7 Antihistamines and decongestants may help relieve nasal allergies and congestion, but they have not been shown to improve healing or reduce the complications of AOM.5 Dosing of analgesics and oral antibiotic agents in pediatric patients is based on weight (kg). Recommended dosing ranges are given in TABLE 2.
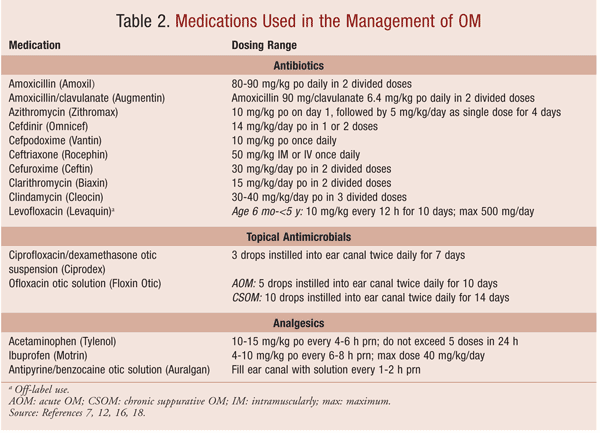
Since episodes of AOM frequently are associated with pain, the use of analgesics—especially during the first 24 hours of an episode—are strongly recommended by the AAP/AAFP guidelines. Treatment options to reduce the pain associated with otalgia include acetaminophen, ibuprofen, and antipyrine/benzocaine otic solution.7 Antipyrine/benzocaine otic solution is a prescription product used for its local analgesic properties. After the solution is instilled into the ear canal, relief of ear pain occurs in approximately 30 minutes. This product is for otic use only, and it should not be used if the solution is brown or contains a precipitate. Disposal of the bottle is recommended 6 months after the dropper is placed in the solution.12
Treatment with an antibacterial agent is recommended for children younger than 6 months of age, children aged 6 months to 2 years with a certain diagnosis of AOM, and any child with moderate-to-severe otalgia or a fever of 102.2°F (39°C) or greater. Observation without antibacterial treatment may be considered for healthy children aged 6 months to 2 years with nonsevere illness and an uncertain diagnosis, as well as for children older than 2 years without severe symptoms or with an uncertain diagnosis for 48 to 72 hours; however, appropriate measures should be taken to allow caregiver–physician communication, reevaluation, and access to antibacterial therapy. A child being managed by observation would receive symptomatic treatment only. This recommendation is based on available evidence demonstrating that children who do not receive antibacterial treatment do well and experience no adverse events.7
High-dose amoxicillin is the recommended first-line treatment for children with AOM because of its efficacy and safety, low cost, palatability, and narrow microbiologic spectrum.7 Because of the risk of highly resistant organisms, high-dose amoxicillin should not be used in children who received antibiotics in the previous 30 days, those with AOM and purulent conjunctivitis, or those receiving chronic prophylaxis with amoxicillin. This high-risk group should be given initial treatment with high-dose amoxicillin/clavulanate.
Cephalosporins are an alternative treatment for penicillin-allergic children as long as urticaria or anaphylaxis (type I hypersensitivity reaction) did not occur upon exposure to the penicillin. In children with a history of a penicillin-induced type I hypersensitivity reaction, clindamycin or the macrolide antibiotics azithromycin and clarithromycin may be used. Although high-dose amoxicillin is recommended as first-line therapy, data from pooled analyses did not find any particular antibiotic to have superior efficacy over other antibiotics. Antibiotics included in the comparison studies were penicillin, ampicillin, amoxicillin, amoxicillin/clavulanate, cefaclor, cefixime, ceftriaxone, azithromycin, and trimethoprim/sulfamethoxazole.13
The safety and efficacy of fluoroquinolones have been evaluated to determine the role of these agents in the treatment of OM. Studies have shown that fluoroquinolones may be an option for the treatment of multidrug-resistant AOM; these agents have been found to be clinically effective and to eradicate pathogens.14 Safety is a primary concern with fluoroquinolone treatment in pediatric patients. Fluoroquinolones have a black box warning that their use is associated with an increased risk of tendonitis and tendon rupture in all age groups; however, bone or joint cartilage toxicity appears to occur in animals, without a profound effect in humans.12,14
The standard duration of treatment is 10 days, with a short course being 1 to 7 days.7 Unfortunately, the most appropriate duration of antibiotic treatment has not been established, owing to limitations associated with the studies evaluating this; however, for children aged less than 6 years and all children with severe disease, the recommended treatment duration is 10 days. For children aged 6 years and older with mild-to-moderate AOM, a 5- to 7-day course of antibiotics is appropriate.7 A 2000 Cochrane review of 30 trials concluded that, in otherwise healthy children without recurrent ear infections, a 5-day course of antibiotics was as effective as the 10-day course supported in the AAP/AAFP guidelines.15
Clinical improvement should be evident 48 to 72 hours after beginning initial AOM management. Children who do not respond to first-line therapy with high-dose amoxicillin should be given high-dose amoxicillin/clavulanate. Children who do not improve after initial treatment with high-dose amoxicillin/clavulanate should be switched to ceftriaxone (either intravenously or intramuscularly) daily for 3 consecutive days.7
Topical antibiotics are approved for use in children with tympanostomy tubes who are experiencing AOM and in children suffering from CSOM. The recommended treatment for patients with tympanostomy tubes who have AOM caused by susceptible stains of S aureus, S pneumoniae, H influenzae, M catarrhalis, or P aeruginosa is ciprofloxacin hydrochloride/dexamethasone otic suspension or ofloxacin otic solution. Ofloxacin otic solution may also be used to treat CSOM in children aged 12 years and older. In CSOM, ofloxacin otic solution is recommended for the treatment of susceptible strains of S aureus, P mirabilis, or P aeruginosa. Since ofloxacin otic solution is sterile, it can be used to treat otic infections even when the membrane is perforated, unlike the unsterile ciprofloxacin steroid combination.11,12 Caregiver counseling regarding the proper administration of otic preparations is important (TABLE 3).

Surgical Options
Surgical placement of tympanostomy tubes is recommended for children with recurrent episodes of AOM. The American Academy of Otolaryngology recommends that tympanostomy tubes be considered in children who have had four episodes of AOM in 6 months or six episodes in 1 year.17 Placement of tympanostomy tubes also is suggested for OME. Tympanostomy tubes usually last 6 to 12 months; once extruded, there is no evidence of ongoing benefit.9
Conclusion
The role of the pharmacist in the management of pediatric OM includes ensuring appropriate weight-based dosing of antibiotic and/or analgesic agents and educating caregivers. Counseling regarding the disease state, proper otic administration, completion of antibiotic treatment, and adverse events associated with medication is essential for achieving improvement in the patient’s health.
REFERENCES
1. McCaig LF, Besser RE, Hughes JM. Trends in antimicrobial prescribing rates for children and adolescents. JAMA. 2002;287:3096-3102.
2. The use of antibacterials in paediatric patients with acute otitis media depends on patient age and disease severity. Drugs Ther Perspect. 2008;1:13-16.
3. Powers J. Diagnosis and treatment of acute otitis media: evaluating the evidence. Infect Dis Clin North Am. 2007;21(2):409-426.
4. Pelton S, Leibovitz E. Recent advances in otitis media. Pediatr Infect Dis J. 2009; 28(suppl 10):S133-S137.
5. Ramakrishnan K, Sparks RA, Berryhill WE. Diagnosis and treatment of otitis media. Am Fam Physician. 2007;76:1650-1658.
6. Kerschner JE. Otitis media. In: Kliegman RM, Behrman RE, Jenson HB, Stanton BF, eds. Nelson Textbook of Pediatrics. 18th ed. Philadelphia, PA: Saunders Elsevier; 2007:2632-2645.
7. American Academy of Pediatrics and American Academy of Family Physicians Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics. 2004;113:1451-1465.
8. Natal BL, Chao JH. Otitis media. Emedicine. http://emedicine.medscape.com/article/764006-overview. Accessed December 14, 2009.
9. Morris PS, Leach AJ. Acute and chronic otitis media. Pediatr Clin N Am. 2009;56:1383-1399.
10. American Academy of Family Physicians, American Academy of Otolaryngology-Head and Neck Surgery, and American Academy of Pediatrics Subcommittee on Otitis Media with Effusion. Otitis media with effusion. Pediatrics. 2004;113:1412-1429.
11. Gould JM, Rodgers GL. Topical antimicrobial agents. In: Long SS, Pickering LK, Prober CG, eds. Principles and Practice of Pediatric Infectious Diseases. 3rd ed. Philadelphia, PA: Churchill Livingstone; 2008.
12. Lexi-Comp Online. http://online.lexi.com/crlsql/servlet/crlonline [by subscription]. Accessed December 14, 2009.
13. Takata GS, Chan LS, Shekelle P, et al. Evidence assessment of management of acute otitis media: I. The role of antibiotics in treatment of uncomplicated acute otitis media. Pediatrics. 2001;108:239-247.
14. Arguedas A, Sierra H, Soley C. Fluoroquinolones in pediatrics. Curr Drug Therapy. 2006;1:117-125.
15. Kozyrskyj AL, Hildes-Ripstein GE, Longstaffe SE, et al. Short course antibiotics for acute otitis media. Cochrane Database Syst Rev. 2000;(2):CD001095.
16. Micromedex Healthcare Series. www.thomsonhc.com [by subscription]. Accessed December 14, 2009.
17. Inglis AF, Gates GA. Acute otitis media and otitis media with effusion. In: Cummings CW, Flint PW, Harker LA, et al, eds. Cummings Otolaryngology: Head & Neck Surgery. 4th ed. Philadelphia, PA: Elsevier Mosby, Inc.; 2005.
18. Facts & Comparisons Online. http://online.factsandcomparisons.com [by subscription]. Accessed December 14, 2009.
19. Krypel L. Otic disorders. In: Berardi RR, Kroon LA, McDermott JH, et al, eds. Handbook of Nonprescription Drugs. 15th ed. Washington, DC: APhA Publications; 2006.
To comment on this article, contact rdavidson@uspharmacist.com.





