US Pharm. 2024;49(5):17-21.
ABSTRACT: Alcohol use disorder is a chronic brain disorder that is characterized by uncontrolled dependence on alcohol and has impacted more than 14.5 million people in the United States. Alcohol-related deaths are increasing. There are many nonpharmacologic and pharmacologic agents available for treatment. Pharmacists have a role in selecting appropriate treatment options and providing education to patients.
According to the 2022 National Survey on Drug Use and Health, individuals aged 18 years and older reported that 52.9% drank alcohol in the past month, while 84.1% drank alcohol at least sometime in their lifetime.1 In 2022, 29.5 million people aged 12 years and older in the United States had alcohol use disorder (AUD), while 7.6% of those with AUD received treatment within the past year.1 Approximately 178,000 people die each year in the U.S. due to an alcohol-related cause, while 2.5 million deaths occur annually worldwide due to harmful use.1,2
In the U.S., alcohol is considered the third-leading preventable cause of death.1 Alcohol misuse is defined as drinking that could cause harm to the individual or surrounding persons or drinking by anyone who is younger than the legal drinking age of 21 years.1 “Binge drinking” is defined by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) as drinking that causes blood alcohol concentration levels of 0.08 g/dL or higher, while the Substance Abuse and Mental Health Services Administration defines it as consuming five or more alcoholic drinks for males or four or more for females on one occurrence.1
Within the U.S., a “standard” drink is considered one of the following: 12 ounces of regular beer that contains approximately 5% alcohol by volume (ABV), 5 ounces of wine that contains approximately 12% ABV, or 1.5 ounces of distilled spirits that contain approximately 40% ABV. Each standard drink contains approximately 14 g of pure alcohol.3 The NIAAA considers heavy drinking for men as consuming more than 14 drinks per week or four drinks a day and women more than seven drinks per week or three drinks a day.1 Drinking these amounts of alcohol puts an individual at an increased risk of health consequences.
RISK FACTORS
Risk factors for developing AUD are summarized in TABLE 1. Individuals with other substance use disorders (SUDs) are at higher risk of developing AUD.4 Individuals with certain genes may be more susceptible to alcoholic liver disease or other alcohol-related comorbidities.
The exact pathogenesis of AUD is not known, but it is believed that its development is multifactorial due to genetics, environmental influences, cognitive function, and personality traits.4

SCREENING/DIAGNOSIS
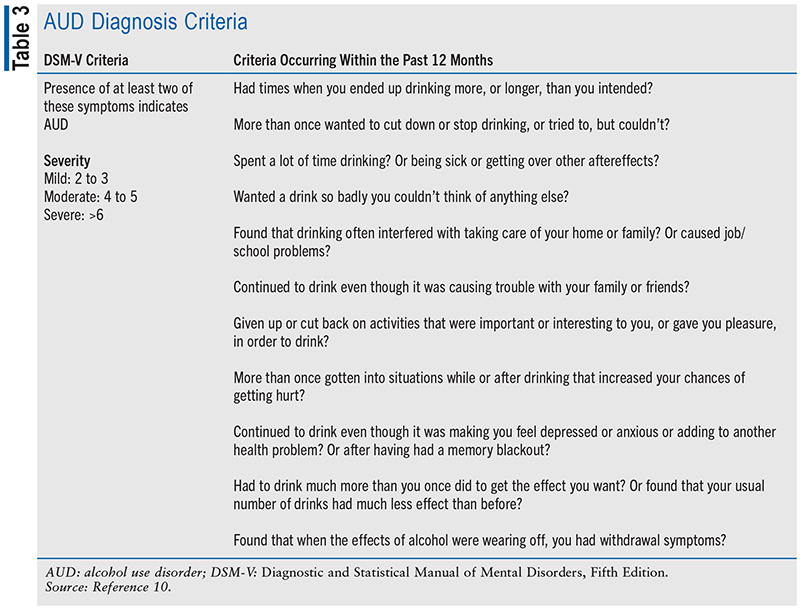
The goals of screening for AUD are to identify patients who are at risk and pave the way for further assessment, diagnosis, and treatment.5 The U.S. Preventative Services Task Force recommends that clinicians screen adults for alcohol misuse and provide those engaged in risky drinking interventions to reduce alcohol misuse.6 There are various screening tool questionnaires available for AUD, but the three most widely used are the Alcohol Use Disorders Identification Test (AUDIT), the Alcohol Use Disorders Identification Test Concise (AUDIT-C), and the CAGE questionnaire (see TABLE 2). AUDIT—a 10-item screening tool created by the World Health Organization—is considered the “gold standard” for alcohol screening.7 Scores range from 0 to 40, with different scores categorizing patients into different “zones” that indicate specific interventions.5 The AUDIT-C is a condensed version of the AUDIT that contains three multiple-choice questions scored from 0 to 4 points.8 In men, a score of 4 or more and in women a score of 3 or more suggests alcohol misuse and the need for further assessment.8 The CAGE questionnaire comprises four “yes” or “no” questions and focuses on the consequences of drinking.9 A “yes” to one or more questions indicates the need for further assessment.9 AUD, like other SUDs, is diagnosed using the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (see TABLE 3).10

CLINICAL MANIFESTATIONS
AUD can affect numerous organ systems, leading to many medical consequences. These clinical manifestations include, but are not limited to, neurologic (electrolyte disturbances, seizures); psychiatric (depression, anxiety, other SUDs); cardiovascular (hypertension, arrhythmias); hepatic (cirrhosis, hepatitis); infectious (sexually transmitted diseases); hematologic (anemia, bone marrow suppression); endocrine (hypoglycemia or hyperglycemia); various malignancies; and other health consequences, such as injury, trauma, and sleep disturbances.4
Individuals with unhealthy alcohol use may present asymptomatically or with signs and symptoms that are not related to alcohol use, including hypertension, gastrointestinal reflux, or sleep disturbances. Individuals with AUD may present in acute alcohol intoxication—characterized by slurred speech, nystagmus, unsteady gait, hypotension, tachycardia—or with symptoms of withdrawal, including hallucinations, seizures, or tremors.4
TREATMENT
The initial goals of treatment of AUD should be agreed upon between the patient and the clinician and may include abstinence, reduction, or moderation of alcohol use, reduction of heavy drinking, or other harm-reduction strategies.11
Nonpharmacologic Treatment
The American Psychiatric Association recommends that patient-centered treatment plans for AUD should include nonpharmacologic and pharmacologic treatments.11 All patients with AUD should be encouraged to participate in some type of behavioral treatment, as it can help patients identify and change behaviors associated with drinking.12 Behavioral treatments help patients develop skills needed to minimize drinking, build strong social support systems, and cope with triggers that may cause them to return to use.12 Clinical trials have not found any behavioral treatment to be superior to others.12 Some behavioral treatments may include motivational enhancement therapy (MET), cognitive behavioral therapy (CBT), couples and family counseling, and community-based peer support groups, such as Alcoholics Anonymous and 12-Step.12,13
MET involves motivational interviewing to strengthen motivation and to build a plan to change drinking behaviors.13 MET focuses on helping patients identify the pros and cons of seeking treatment and developing the skills necessary to change drinking behaviors.13 CBT focuses on identifying the relationships between feelings, thoughts, and behaviors that lead to heavy drinking.13 The goal with CBT is to help patients manage stress and develop the skills necessary to cope with triggers that may lead them to return to use.13 Studies have shown that strong family support increases alcohol abstinence, highlighting the importance of social support groups.13
Pharmacologic Treatment
There are three pharmacologic agents that are currently FDA approved for the treatment of AUD: naltrexone, acamprosate, and disulfiram.11,13 The American Psychiatric Association recommends using naltrexone or acamprosate in patients with moderate-to-severe AUD as first-line therapies.11 It recommends disulfiram, immediate-release (IR) topiramate, or gabapentin as second-line therapies for patients who are intolerant or have not responded to naltrexone and acamprosate.11 Dosing, adverse effects, and selected clinical pearls for naltrexone, acamprosate, disulfiram, topiramate IR, and gabapentin are summarized in TABLE 4.

The oral formulation of naltrexone was FDA approved for the treatment of AUD in 1994, while the IM formulation was approved in 2006.14 Naltrexone is a nonselective competitive antagonist of opioid receptors, primarily the µ-opioid receptors.15,16 Blocking the opioid receptors may diminish the reward and reinforcing effects from dopamine, which may reduce alcohol consumption.15,16 Naltrexone may also modify the hypo-pituitary-adrenal axis to suppress alcohol consumption.15,16 Limited evidence suggests that while both oral and IM formulations of naltrexone appear to be effective for the treatment of AUD, long-acting injectable naltrexone may offer improved compliance compared with oral formulations, although direct comparisons between the two are lacking.11
Acamprosate was approved by the FDA in 2004 for the treatment of AUD.14 Acamprosate is structurally similar to gamma-aminobutyric acid (GABA), and it appears to increase the activity of the GABA-ergic system and decrease the activity of glutamate within the central nervous system.17 Patients with AUD may have unbalanced GABA and glutamate activities, which may be restored by acamprosate.17 Of note, medication compliance may be an issue with acamprosate due to the high pill burden, in which patients must take two 333-mg tablets three times a day for a total of six tablets daily.
Disulfiram became the first FDA-approved drug for the treatment of AUD in 1948.14 Disulfiram is a thiuram derivative that blocks the oxidation of alcohol at the acetaldehyde stage.18 When taken with alcohol, it causes uncomfortable symptoms including flushing, throbbing in head and neck, nausea, vomiting, diaphoresis, palpitations, chest pain, dyspnea, hyperventilation, tachycardia, syncope, weakness, blurred vision, confusion, vertigo, and hypotension.18 This disulfiram-alcohol reaction may occur up to 14 days after discontinuing disulfiram. Of note, hepatotoxicity has occurred with the use of acamprosate, including hepatitis and hepatic failure, and patients should be monitored and educated about signs and symptoms.18
Topiramate and gabapentin are not FDA approved for the use of AUD but may be used off-label.11 Topiramate enhances GABA(A) activity and antagonizes α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate glutamate receptors.19 Gabapentin is a GABA analogue and has high affinity for gabapentin-binding sites throughout the brain, which may modulate the release of excitatory neurotransmitters.20
MANAGEMENT OF ALCOHOL WITHDRAWAL
Abrupt cessation of alcohol can cause anxiety, agitation, tremors, or more severe symptoms like seizures and delirium tremens and generally correlates to alcohol intake and duration of drinking habit.21 The Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar) scale is a 10-item assessment tool that shows the severity of alcohol withdrawal and can be used to monitor and assist with treatment of patients in withdrawal.21 The cumulative score of 8 or less indicates mild withdrawal, 8 to 15 denotes moderate withdrawal, and scores of 15 or greater correlate with severe withdrawal.21,22 When assessing a patient, the full clinical picture should be considered when determining treatment. Patients with moderate or severe alcohol withdrawal need close monitoring and may need to be treated in the ICU.
Electrolyte levels should be monitored and repleted as needed. IV fluids may be needed to treat dehydration. Nutritional supplementation may be needed to manage nutritional deficits. Thiamine and glucose are administered to prevent or treat Wernicke encephalopathy. Frequent clinical reassessments including vital signs are ongoing.21
Benzodiazepines are used to treat psychomotor agitation during withdrawal and to prevent progression of withdrawal symptoms. Diazepam, lorazepam, and chlordiazepoxide are commonly used. The choice of therapy is dependent on pharmacokinetics.21,23
There are different treatment regimens that can be utilized for alcohol withdrawal, including front-loading, fixed-schedule, and symptom-triggered regimens. Front loading is appropriate for patients who are at greater risk for experiencing dangerous complications should they develop severe withdrawal. Diazepam 5 mg to 10 mg can be administered IV every 5 to 10 minutes until the appropriate level of sedation occurs. An alternative to diazepam is lorazepam 2 mg to 4 mg IV every 15 to 20 minutes.21,23 Fixed-schedule therapy is when benzodiazepine is given at set intervals even if symptoms are not present. This method prevents withdrawal in patients who are at risk for withdrawal but are asymptomatic or have minimal symptoms.21,23 The patient should not continue to receive benzodiazepine if he or she is sedated. In symptom-triggered regimens, medication is only provided based on symptoms. The CIWA-Ar can be administered every 10 to 15 minutes in those with more severe symptoms and then followed by an hourly reassessment. Those who have mild symptoms may be assessed every 4 to 6 hours.21,23 The CIWA-Ar of 8 to 10 points indicates treatment.
PHARMACIST’S ROLE
Pharmacists have the opportunity to provide evidence-based pharmacotherapy recommendations and psychosocial interventions as a member of a multidisciplinary team to treat individuals with AUD. Pharmacists can monitor pharmacotherapy for effectiveness and potential side effects or drug interactions. In settings with established protocols, pharmacists can participate in the dispensing and administration of IM naltrexone as part of the treatment for AUD. Pharmacists can encourage patients to obtain treatment for AUD.24,25
CONCLUSION
AUD is common and has a significant impact on an individual’s life. Patient-centered treatment plans should include support groups and pharmacologic agents as appropriate. Pharmacists have a role in selecting appropriate treatment options and providing education to patients.
REFERENCES
1. National Institute on Alcohol Abuse and Alcoholism. Alcohol facts and statistics. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics. Accessed February 26, 2023.
2. Wackernah RC, Minnick MJ, Clapp P. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil. 2014;5:1-12.
3. National Institute on Alcohol Abuse and Alcoholism. What is a standard drink? www.niaaa.nih.gov/alcohols-effects-health/overview-alcohol-consumption/what-standard-drink. Accessed February 20, 2023.
4. Tetrault JM, O’Connor PG. Risky drinking and alcohol use disorder: epidemiology, pathogenesis, clinical manifestations, course, assessment, and diagnosis. UpToDate [Internet]. Waltham, MA: UpToDate, Inc. www.uptodate.com. Accessed February 26, 2023.
5. Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care. 2nd ed. Geneva, Switzerland. World Health Organization; 2001.
6. U.S. Preventive Services Task Force; Curry SJ, Krist AH, Owens DK. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;320(18):1899-1909.
7. CDC. Alcohol screening and brief intervention for people who consume alcohol and use opioids. August 5, 2022. www.cdc.gov/alcohol/fact-sheets/alcohol-screening.html. Accessed February 26, 2023.
8. Bradley KA, DeBenedetti AF, Volk RJ, et al. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31(7):1208-1217.
9. Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252(14):1905-1907.
10. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Arlington, VA: American Psychiatric Association; 2013.
11. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. Am J Psychiatry. 2018;175(1):86-90.
12. Holt SR. Alcohol use disorder: treatment overview. UpToDate [Internet]. Waltham, MA: UpToDate, Inc. www.uptodate.com. Accessed February 26, 2023.
13. National Institute on Alcohol Abuse and Alcoholism. Treatment for alcohol problems: finding and getting help. September 2023. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/treatment-alcohol-problems-finding-and-getting-help. Accessed April 4, 2024.
14. Zindel LR, Kranzler HR. Pharmacotherapy of alcohol use disorders: seventy-five years of progress. J Stud Alcohol Drugs Suppl. 2014;75(Suppl 17):79-88.
15. Naltrexone hydrochloride product information. Congers, NY: Chartwell Rx, LLC; December 2021.
16. Vivitrol (naltrexone) product information. Waltham, MA: Alkermes, Inc; September 2022.
17. Campral (acamprosate) product information. St. Louis, MO: Forest Laboratories, Inc; January 2012.
18. Disulfiram product information. Bensalem, PA: Sigmapharm Laboratories LLC; March 2021.
19. Topamax (topiramate) product information. Titusville, NJ: Janssen Pharmaceuticals, Inc; October 2022.
20. Neurontin (gabapentin) product information. New York, NY: Parke-Davis; October 2021.
21. Bayard M, Mcintyre J, Hill KR, Woodside J Jr. Alcohol withdrawal syndrome. Am Fam Physician. 2004;69(6):1443-1450.
22. American Society of Addition Medicine. Addiction medicine essentials clinical institute withdrawal assessment of alcohol scale, revised (CIWA-Ar). www.ci2i.research.va.gov/paws/pdfs/ciwa-ar.pdf Accessed April 18, 2024.
23. Hoffman RS, Weinhouse GL. Management of moderate and severe alcohol withdrawal syndromes. UpToDate [Internet]. www.uptodate.com/contents/management-of-moderate-and-severe-alcohol-withdrawal-syndromes#H192. Accessed April 4, 2024.
24. American Society of Health-System Pharmacists. ASHP statement on the pharmacist’s role in substance abuse prevention, education, and assistance. Am J Health-Syst Pharm. 2016;73:e267-70.
25. University of Wisconsin-Madison School of Pharmacy. Steps to initiating a pharmacy-based injectable naltrexone service. www.pharmacy.wisc.edu/faculty/ford-research-group/resources/injectable-naltrexone-best-practices/steps-to-initiating-a-pharmacy-based-injectable-naltrexone-service/?doing_wp_cron=1712008539.6891050338745117187500. Accessed April 1, 2024.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





