US Pharm. 2018;43(7):HS-13-HS-16.
ABSTRACT: Healthcare professionals across the world utilize the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guideline to guide the diagnosis, management, and prevention of chronic obstructive pulmonary disease (COPD). The guideline incorporates evidence-based recommendations regarding the assessment of disease severity, choice of pharmacologic treatment, and strategies for the management and prevention of acute exacerbations. The GOLD guideline recently underwent a major revision in 2017, in addition to a minor revision in 2018, to account for new evidence surrounding the assessment of disease severity, as well as therapeutic recommendations for the management of COPD. The updated GOLD report includes a simplified version of the ABCD assessment tool, which separates symptoms and exacerbation risk from the severity of airflow limitation. Additionally, there were also modifications to the pharmacotherapy treatment algorithm and new recommendations for the prevention and management of acute COPD exacerbations.
Since 2001, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) has released guidelines to provide clinicians with the tools they need to properly and consistently diagnose, manage, and prevent COPD. The GOLD report defines COPD as a preventable disease characterized by progressive airflow limitation and persistent respiratory symptoms.1-3 Tobacco smoke is one of the greatest risk factors for the development of COPD.
Aside from tobacco smoke, exposure to noxious particles from the environment and various host factors, including genetics, age, and airway hyper-responsiveness, also influence disease development.3 The World Health Organization projects that by the year 2030, COPD will be the third-leading cause of death worldwide owing to an increase in risk-factor exposure and the aging of the world’s population.3,4
The management of COPD depends on the assessment of disease severity. The degree of chronic airflow limitation is measured by spirometry and progresses at varying rates over time, differing from person to person.3 As the lungs are exposed to noxious particles or gases, they become inflamed. Over time, chronic inflammation causes structural changes to the airway, resulting in progressive airflow limitation seen upon spirometry.3 The structural narrowing of the peripheral airways, in addition to the chronic inflammation, is directly related to the reduction in the volume of air exhaled at the end of the first second of forced expiration (FEV1) typically seen in patients with COPD.3,5
A diagnosis of COPD, therefore, should be considered in patients with a prior history of risk-factor exposure, in addition to symptom development such as dyspnea, chronic cough, or sputum production.3 To establish an official diagnosis of COPD in a patient with risk factors and symptoms, a postbronchodilator FEV1 to forced vital capacity (FVC) ratio (FEV1/FVC) < 0.70 is required to confirm the presence of airflow limitation utilizing spirometry.2,3 The 2018 GOLD report emphasizes the need to perform an additional spirometry test at a later date if the FEV1/FVC ratio value is between 0.6 and 0.8 to account for variation in measurements.3 The updated guideline also no longer recommends measuring FEV1 before and after a bronchodilator in an attempt to assess the degree of airflow limitation reversibility, as it provides no additional benefit in the diagnosis or management of COPD.2,3
Assessment of COPD
The assessment of COPD is imperative for guiding therapy and contains three major components: classification of airflow limitation, severity of symptoms, and exacerbation history. The use of the spirometric grading system was previously utilized to assess disease severity until it was replaced in 2011 with the ABCD assessment tool. This assessment tool aimed to incorporate a triad of spirometric testing, degree of symptom burden, and exacerbation risk into the assessment of the disease to help guide medication therapy. Studies have shown there is little correlation between FEV1 and the health status of a patient.6 Thus, FEV1 should not be used alone to guide individual treatment recommendations.
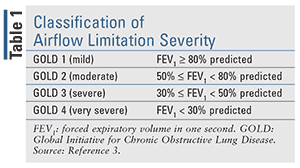
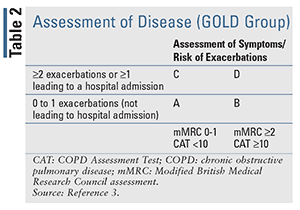
In 2017, the GOLD report separated symptoms and exacerbation history from the severity of airflow limitation in the assessment of disease severity to emphasize the clinical parameters that drive therapeutic recommendations.2 The revised assessment tool allows for the initiation of treatment based upon the assessment of symptoms and history of exacerbation only, while the assessment of airflow limitation remains separate. The classification of airflow limitation severity in patients with COPD (confirmed by FEV1/FVC < 0.70) can be seen in TABLE 1. This revised assessment tool (TABLE 2) makes it easier for clinicians to begin treatment based on the patient’s previous 12-month history of exacerbations and symptom assessment utilizing the Modified British Medical Research Council (mMRC) questionnaire or the COPD Assessment Test (CAT).3
Pharmacotherapy for Stable COPD
Identifying and eliminating risk factor exposure is crucial for the management of COPD. At each visit, smoking cessation should be addressed, and all patients who smoke should be encouraged to quit. Upon assessment of disease severity, therapy should be initiated based upon the patient’s symptoms and exacerbation history. The GOLD guideline supports a treatment algorithm (TABLE 3) that can be used to escalate or de-escalate therapy based upon a patient’s current GOLD Group. For patients in GOLD Group A, a bronchodilator (short- or long-acting) should be provided (see TABLE 4 online at www.uspharmacist.com). For patients in Group B, a long-acting bronchodilator with either a long-acting beta-agonist (LABA) or a long-acting muscarinic antagonist (LAMA) should be initiated (TABLE 4). There is no evidence for recommending one over the other aside from patient preference. If symptoms persist, a LAMA with a LABA (TABLE 4) can be used in conjunction. Initial therapy for patients in Group C should consist of a LAMA over a LABA, as two previous trials demonstrated the superiority of a LAMA over a LABA.7,8 For patients in Group C with persistent exacerbations despite LAMA use, combination therapy of LABA with LAMA may be beneficial.3
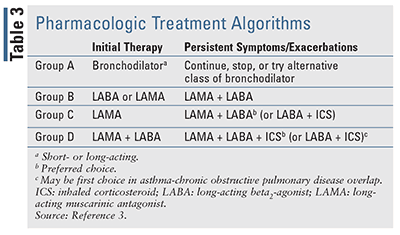
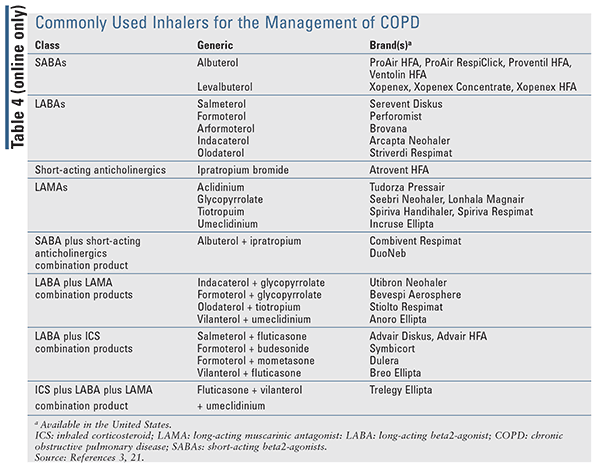
Finally, for patients in GOLD Group D (TABLE 3), initial therapy should consist of a LABA plus LAMA combination (TABLE 4). There is no role for inhaled corticosteroids (ICS) monotherapy in the treatment of COPD due to the lack of mortality benefit and failure to prevent further reductions in FEV1 over time. Furthermore, the GOLD report suggests that combination therapy with ICS/LABA may be a viable option for patients with high blood eosinophil counts or a history of asthma-COPD overlap.3 For patients on dual LABA/LAMA therapy in Group D who continue to have exacerbations, escalation to triple therapy with a LABA/LAMA/ICS product may be appropriate. Two randomized, controlled trials showed clinical benefit of triple therapy compared with LAMA alone or ICS/LABA therapy in patients with advanced disease.9,10
Trelegy Ellipta, a once-daily triple therapy of fluticasone furoate, umeclidinium, and vilanterol, was approved by the FDA in September 2017 for the management of COPD in patients requiring additional bronchodilation while receiving Breo Ellipta (fluticasone furoate/vilanterol) with or without Incruse Ellipta (umeclidinium). Recently, the FDA expanded the indication for Trelegy Ellipta to a broader COPD population that includes airflow limitation and acute symptoms worsening.11 If exacerbations continue despite triple therapy, the addition of roflumilast may be considered in Group D patients with an FEV1 <50% predicted, especially if they have had a previous hospitalization for a COPD exacerbation within the last 12 months.3,12-14 Therapy can also be de-escalated over time depending upon assessed disease severity.
Managing COPD Exacerbations
A COPD exacerbation is defined as an acute worsening of dyspnea and other symptoms (e.g., increased sputum and mucus production and/or purulence, and/or coughing and wheezing) that require additional therapy. Increased airway inflammation and gas trapping may also worsen symptoms.1-3 The three cardinal symptoms of COPD exacerbation include increases in dyspnea, sputum volume, and sputum purulence. Though symptoms of COPD exacerbations usually last for about 7 to 10 days, the patient may not fully recover for several weeks to months.3
Risk factors associated with developing an exacerbation include duration of COPD, history of antibiotic or theophylline use, advanced age, increased ratio of pulmonary artery to aorta cross-sectional dimension, and comorbid conditions (e.g., chronic heart failure, diabetes mellitus, etc.). Patients with increased sputum production, productive cough, and an elevated blood eosinophil count (>0.34 x 109 cells/L) are also at increased risk for COPD exacerbations.3,15 The majority of exacerbations result from respiratory infections caused by virus (e.g., human rhinovirus) and bacteria (e.g., Haemophilus influenzae, Moraxella catarrhalis, Streptococcus pnuemoniae, and Pseudomonas aeruginosa).15,16 Environmental pollution, temperature, and pulmonary embolism are also known exacerbation triggers.3
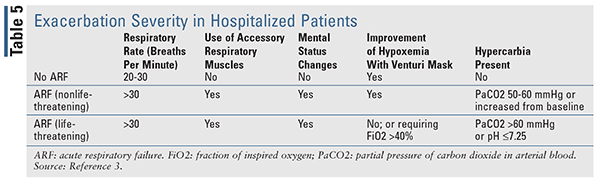
Mild and moderate COPD exacerbations may be managed in the outpatient setting, whereas severe exacerbations should be managed in the emergency department and sometimes require hospitalization, such as those with onset of new cyanosis, peripheral edema, worsening dyspnea at rest, a high respiratory rate, decreased oxygenation saturation, confusion, or drowsiness. Patients with serious comorbidities (e.g., heart failure, arrhythmias, etc.), acute respiratory failure, insufficient home support, and those who fail initial medical management should also be managed as inpatients. Furthermore, admission to the intensive care unit should be considered for patients with mental status changes; who are hemodynamically unstable; or who are experiencing severe dyspnea, persistent or worsening hypoxemia, and/or severe or worsening respiratory acidosis despite initial therapy, supplemental oxygen, and noninvasive ventilation. The exacerbation severity in hospitalized patients should be assessed based on the patient’s clinical signs (TABLE 5).3
The standard treatment for COPD exacerbations include bronchodilators (e.g., SABA, anticholinergics), corticosteroids, and antibiotics (TABLE 6).3,16,17 Supplemental oxygen should also be initiated and titrated to achieve an oxygen saturation of 88% to 92%.3 As an alternative to oxygen therapy, oxygen via high-flow nasal cannula or noninvasive positive pressure ventilation can also be used to improve oxygenation and ventilation and decrease hypercarbia in acute hypoxemic respiratory failure.3
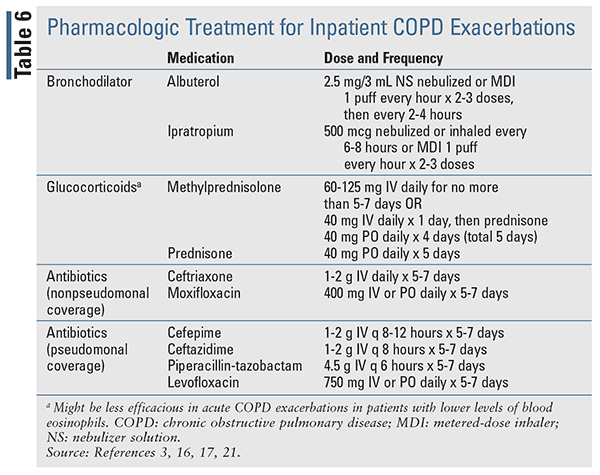
Since many COPD exacerbations can be caused by viruses, antibiotics are controversial and are only recommended for 5 to 7 days for the following indications, which suggest a bacterial infection: when a patient presents with all three of the cardinal symptoms, or with increased sputum purulence plus one of the other cardinal symptoms, or if the patient is mechanically ventilated (either invasive or noninvasive). Some studies have demonstrated that antibiotics can decrease the risk of short-term mortality, treatment failure, and sputum purulence in at least moderately severe patients with a COPD exacerbation. Biomarkers, such as C-reactive protein, may also be used to support a suspected bacterial infection; however, this is a nonspecific marker and its utility as a biomarker is controversial.
Recent literature investigating procalcitonin as a biomarker for infection has shown positive results in being more specific for bacterial infections and positively guiding antibiotic decision use/de-escalation.3,18-20 Normal serum procalcitonin is <0.1 ng/mL in humans, and elevated concentrations indicate the likelihood of a bacterial infection. The GOLD report suggests considering utilization of procalcitonin-based protocols to guide antibiotic use as studies have shown an association with procalcitonin use and decreased antibiotic prescription and total antibiotic exposure.3
COPD exacerbations can negatively impact disease progression and a patient’s health status. Thus, minimizing the number of exacerbations by adhering to long-term chronic management strategies and preventative maintenance therapy should be a key goal in the chronic management of COPD. Regimens containing LABAs and LAMAs, as monotherapy or in combination with each other and/or corticosteroids, have been proven to reduce the frequency of COPD exacerbations. Roflumilast, in combination with systemic corticosteroids, has also been shown to reduce moderate and severe exacerbations.12-14
REFERENCES
1. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2016 Report). http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016. Accessed April 14, 2018.
2. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2017 Report). http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed April 14, 2018.
3. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2018 Report). http://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-revised-20-Nov_WMS.pdf. Accessed April 14, 2018.
4. World Health Organization. Global Burden of Disease. 2018. www.who.int/respiratory/copd/burden/en/. Accessed April 14, 2018.
5. Hogg JC, Chu F, Utokaparch S, et al. The nature of the small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645-2653.
6. Jones PW. Health status and the spiral of decline. COPD. 2009;6(1):59-63.
7. Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364(12):1093-1103.
8. Decramer ML, Chapman KR, Dahl R, et al. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomized, blinded, parallel-group study. The Lancet Respiratory Medicine. 2013;1(7):524-533.
9. Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomized controlled trial. Lancet. 2017;389(10082):1919-29.
10. Lipson DA, Barnacle H, Birk R, et al. FULFIL Trial: once-daily triple therapy for patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(4):438-446.
11. FDA expands indication for Trelegy Ellipta in COPD. Medscape. 2018. www.medscape.com/viewarticle/895665?src=wnl_edit_newsal_180425_MSCPEDIT&uac=149751ST&impID=1616131&faf=1. Accessed April 14, 2018.
12. Martinez FJ, Calverley PM, Goehring UM, et al. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomized controlled trial. Lancet. 2015;385(9971):857-866.
13. Rabe KF, Calverley PMA, Martinez FJ, et al. Effect of roflumilast in patients with severe COPD and a history of hospitalization. Eur Respir J. 2017;50(1).
14. Martinez FJ, Rabe KF, Sethi S, et al. Effect of Roflumilast and inhaled corticosteroid/long-acting beta-2-agonist on chronic obstructive pulmonary disease exacerbations (RE2SPOND) a randomized clinical trial. Am J Respir Crit Care Med. 2016;194(5):559-567.
15. Papi A, Rabe KF, Rigau D, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Euro Respir J. 2017;49:1600791.
16. Sethi S. Bacteria in exacerbations of chronic obstructive pulmonary disease. Am Thoracic Soc. 2004;1:109.
17. Stoller JK, Barnes PJ, Hollingsworth H. Managment of exacerbations of chronic obstructive pulmonary disease. UpToDate. Last updated April 6, 2018.
18. Schuetz P, Wirz Y, Mueller B. Procalcitonin testing to guide antibiotic therapy in acute respiratory upper and lower respiratory tract infections. JAMA. 2018;319(9):925-926.
19. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;(9):Cd007498.
20. Covington E, Roberts M, Dong J. Procalcitonin monitoring as a guide for antimicrobial therapy: a review of current literature. Pharmacotherapy. 2018;38(5):569-581.
21. Micromedex Solutions. Truven Health Analytics, Inc. Ann Arbor, MI. www.micromedexsolutions.com. Accessed May 8, 2018.
To comment on this article, contact rdavidson@uspharmacist.com.





