US Pharm. 2023;48(11):24-28.
ABSTRACT: Gestational diabetes mellitus (GDM) affects a significant percentage of U.S. pregnancies, rising from 6.0% in 2016 to 8.3% in 2021, with higher rates in older mothers. GDM’s complex causes involve hormonal and metabolic factors leading to insulin resistance, posing risks to both mother and baby. Risk factors include obesity, sedentary lifestyles, prediabetes, polycystic ovary syndrome, family history, and specific ethnic backgrounds. Screening is vital during pregnancy, postpartum, and lifelong for those with GDM history. Management includes blood glucose monitoring, lifestyle changes, and medication when necessary. Pharmacists play a crucial role in medication management and patient education to promote healthier pregnancies and lives.
Gestational diabetes mellitus (GDM) impacts a significant number of pregnancies in the United States, with an estimated incidence ranging from 2% to 10% each year.1 Additionally, type 2 diabetes (T2D) is more likely to advance in approximately 50% of cases for pregnant women who acquire GDM, increasing their risk of developing the condition after delivery. Over time, there has been a concerning increase in the prevalence of GDM. For instance, the percentage of mothers receiving a GDM diagnosis during pregnancy rose from 6.0% in 2016 to 8.3% in 2021.2 This upward trend was observed across all maternal age groups, with rates steadily rising with increasing maternal age.
In 2021, the rate of GDM among mothers aged 40 years or older reached 15.6%, nearly six times higher than the rate for mothers aged younger than 20 years, which stood at 2.7%.2 These statistics highlight the importance of monitoring and addressing GDM to promote the health and well-being of pregnant women and their babies. Early detection and appropriate management strategies are crucial in mitigating the long-term health risks associated with GDM and the potential progression to T2D.
Pathophysiology
GDM is characterized by elevated blood glucose concentrations (BGCs) during pregnancy in women who were not previously diabetic. The pathophysiology involves a complex interplay of hormonal, metabolic, and placental factors that affect insulin sensitivity and secretion. During pregnancy, the placenta plays a crucial role in producing various hormones, including human placental lactogen and progesterone. While these hormones are essential for supporting the fetus’s growth and development, they can also have unintended consequences on the mother’s metabolism. Some of these hormones promote insulin resistance in specific tissues, such as skeletal muscle and adipose tissue, which makes it more difficult for glucose to enter cells and be utilized for energy.3 This physiological insulin resistance ensures a steady supply of glucose to the developing fetus. Still, it becomes more pronounced in some cases, leading to relative insulin deficiency and elevated BGCs. The pancreas attempts to produce more insulin to compensate for the increased insulin resistance.
In some women with GDM, the beta cells within the pancreas may not be able to secrete enough insulin to overcome the resistance, leading to inadequate insulin response and further contributing to hyperglycemia.3 Moreover, the hormonal changes and insulin resistance in GDM can lead to an upsurge in hepatic glucose production by the liver. Therefore, women with GDM undergo increased glucose production, which results in elevated BGCs, raising the complexity of the condition. The liver plays a crucial role in managing BGCs by releasing stored glucose into the bloodstream.
Furthermore, GDM is associated with an impaired incretin effect. In response to eating, the intestine produces incretin hormones, stimulating the pancreas to secrete insulin and promoting effective glucose utilization after meals. In GDM, this incretin effect may be blunted, resulting in reduced insulin secretion following meals and prolonged postprandial hyperglycemia.3 Additionally, functional changes occur in the beta cells of the pancreas during pregnancy. These changes may affect the beta cells’ ability to adapt adequately to the increased insulin requirements, leading to further impairment in insulin secretion.4
Risk Factors
GDM is associated with numerous risk elements, with a higher BMI as one of the most firmly established factors contributing to its development.5 Excess weight can lead to insulin resistance, making it more challenging for cells to respond to insulin appropriately and resulting in elevated BGCs. Additionally, leading a sedentary lifestyle with low physical activity can exacerbate insulin resistance and contribute to the development of GDM. An essential risk factor for GDM is prediabetes, which entails elevated BGCs beyond the normal range but not reaching the diabetes threshold, and women with prediabetes are more prone to transitioning to GDM during pregnancy.5
The risk of developing GDM in subsequent pregnancies is heightened if a patient experienced GDM during a previous pregnancy.6 Medical conditions such as polycystic ovary syndrome (PCOS) can influence hormonal imbalances and insulin resistance, further raising the risk of GDM. Family history plays a vital role in the development of GDM. Having an immediate family member with diabetes, especially a first-degree relative such as a parent or sibling, increases the risk. The presence of a familial history of diabetes indicates a genetic predisposition to the condition, emphasizing the importance of prompt monitoring and making lifestyle adjustments during pregnancy.
Furthermore, women who have previously delivered large babies weighing more than 9 pounds (4.1 kg) are at an increased risk for GDM in subsequent pregnancies. Inadequately managed BGCs during pregnancy can lead to the birth of larger infants, emphasizing the importance of careful monitoring and control in subsequent pregnancies. Race and ethnicity also contribute to GDM risk. Specific racial and ethnic populations, including but not limited to black, Hispanic, American Indian, and Asian American women, exhibit an elevated susceptibility to the onset of GDM.7 This disparity may be linked to genetic factors and lifestyle differences within these populations. Furthermore, an elevated maternal age is linked to a heightened likelihood of GDM occurrence. As women age, their body’s response to insulin may change, leading to insulin resistance and higher BGCs. Regular screening and early detection become crucial for older pregnant women to manage GDM effectively.
Diagnostic Criteria
Screening for GDM is an important component of prenatal care, aimed at identifying and managing this condition promptly to ensure the well-being of both the mother and the developing fetus. The diagnostic criteria for GDM involve a systematic approach to screening at various stages of pregnancy and postpartum follow-up for women who have experienced GDM. By following these screening recommendations, healthcare providers can effectively detect and manage GDM, reducing the risk of complications and promoting a healthy pregnancy outcome (see TABLE 1).
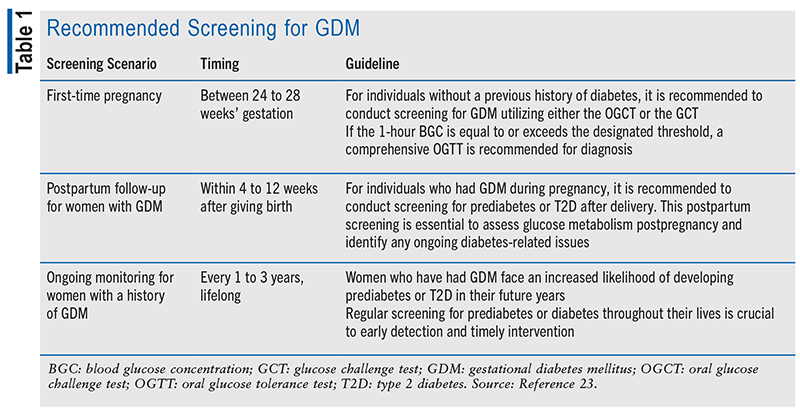
The screening recommendations for GDM are guidelines to serve as vital recommendations for healthcare providers to ensure early detection and effective management of this condition. By performing screening during the 24th to 28th weeks of pregnancy for those without a previous history of diabetes, GDM can be promptly detected and effectively managed. Additionally, postpartum screening within 4 to 12 weeks after giving birth for women with a history of GDM allows for ongoing monitoring and timely intervention.8 Regular screening throughout a woman’s life is also crucial for those who have experienced GDM, as it helps in identifying prediabetes or T2D and addressing diabetes-related risks effectively.
Goals
In managing GDM, a set of specific goals are implemented to ensure optimal maternal and fetal health throughout pregnancy. To effectively control BGCs, women with GDM are advised to follow target ranges for blood glucose testing at different times of the day. The recommended targets are to maintain readings below 95 mg/dL before a meal, 140 mg/dL or less 1 hour after a meal, and 120 mg/dL or less 2 hours after a meal.9 Regularly monitoring BGCs allows for close tracking of glucose control and enables timely adjustments to treatment plans if necessary.
Alongside vigilant blood glucose monitoring (BGM), women are encouraged to adopt a healthy lifestyle that includes making mindful dietary choices.10 Following a well-balanced eating plan is paramount, ensuring that the right amounts of nutrients are consumed at appropriate times. It involves consuming a variety of nutritious foods, focusing on complex carbohydrates, lean proteins, and healthy fats, while limiting sugary and high-calorie options.
Engaging in regular physical activity is another essential component of managing GDM. Moderate-intensity exercises, such as jogging, swimming, or prenatal yoga, have been shown to lower BGCs and enhance insulin sensitivity. Physical activity not only helps regulate blood glucose but also offers additional benefits such as improved cardiovascular health and mood.11
However, despite maintaining a healthy diet and being physically active, some women with GDM may still require additional support to manage their blood sugar effectively. In such cases, healthcare providers may prescribe medications as part of the treatment plan. Insulin therapy is the most common medication used for GDM, and it is considered safe for both the mother and the baby. In some instances, oral medications like metformin may be recommended to help regulate BGCs.12
Treatment
Treatment for GDM involves pharmacologic therapy as one of the options when lifestyle modifications alone are insufficient to control BGCs.13 The three drugs commonly considered for GDM treatment are insulin, metformin, and glyburide. These pharmacologic treatment options for GDM are listed in TABLE 2.
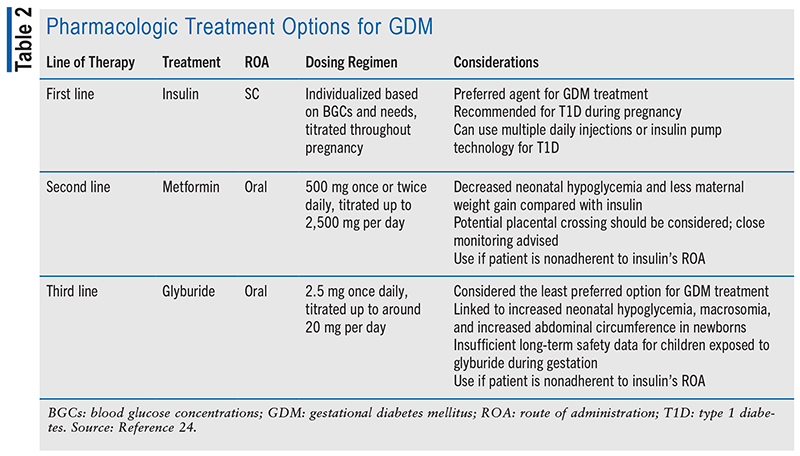
For GDM management, insulin remains the optimal selection, particularly in the context of addressing type 1 diabetes (T1D) during pregnancy. Additionally, it stands as the first-line approach for treating T2D during pregnancy. Insulin can be administered through either multiple daily injections or insulin pump technology in pregnant women with T1D. The advantage of insulin lies in its well-established safety and efficacy, with a long history of use in pregnancy. It allows precise control of BGCs, ensuring optimal maternal and fetal outcomes.14 While insulin therapy may require careful monitoring and dose adjustments, it remains the gold standard for managing GDM due to its proven therapeutic outcome.
Metformin presents an alternative approach for the treatment of GDM, demonstrating potential benefits such as a decreased risk of neonatal hypoglycemia and reduced maternal weight gain, when compared with the use of insulin.15 However, it is essential to be aware that metformin readily crosses the placenta, resulting in concentrations in the umbilical cord blood equivalent to or surpassing those in the maternal bloodstream. As a result, the potential impact of metformin on the developing fetus should be carefully considered and the benefits and risks weighed when choosing a treatment option. Close monitoring and consultation with healthcare professionals are key to ensure the well-being of both the mother and the baby.
Glyburide, a sulfonylurea, is generally the least-preferred drug for GDM treatment. Numerous meta-analyses and systematic reviews have consistently indicated that glyburide is linked to a heightened occurrence of neonatal hypoglycemia, macrosomia, and increased abdominal circumference in newborns compared with insulin or metformin treatments. Moreover, glyburide has not demonstrated noninferiority to insulin regarding a combined outcome measure comprising neonatal hypoglycemia, macrosomia, and hyperbilirubinemia. It is also important to note that glyburide can cross the placenta, potentially raising concerns about its impact on the fetus.16
Postpartum Monitoring
In the postpartum period, managing GDM involves monitoring BGCs and making timely adjustments to insulin dosages during the early postpartum days. This is essential due to insulin requirements typically decreasing by approximately half compared with the pregnancy stage. Healthcare providers should discuss and implement a contraceptive plan with all individuals having diabetes of reproductive potential to facilitate family planning and manage pregnancy-related risks. For recent cases of GDM, it is advisable to conduct screening between 4 to 12 weeks after childbirth. This screening should involve the 75-g oral glucose tolerance test and the utilization of diagnostic criteria applicable outside of pregnancy. Overweight or obese individuals with a history of GDM diagnosed with prediabetes should receive intensive lifestyle interventions and/or metformin for diabetes prevention.12 Encouraging breastfeeding is crucial, as it plays a pivotal role in diminishing the likelihood of maternal T2D. However, blood sugar implications should be considered when choosing between breastfeeding and formula feeding.
Continued postpartum care is critical, with regular screening for T2D or prediabetes recommended throughout life, every 1 to 3 years for those with previous GDM. Individuals with a history of GDM should actively pursue preconception screening and care to identify and manage hyperglycemia and prevent congenital malformations.17 Comprehensive postpartum care should include psychosocial assessment and self-care support that addresses emotional or mental health concerns. Regular monitoring of BGCs and follow-up with physicians are necessary even if some patients do not require insulin postpartum. Regular screenings for T2D should be promoted for long-term health management. Healthcare providers should emphasize blood sugar monitoring, dietary management, physical activity, and medication management, considering prior prescriptions for GDM. For breastfeeding patients, awareness of blood sugar impact is essential, requiring close monitoring and potential diet or medication adjustments.
Pharmacist’s Role
In managing a patient with GDM, pharmacists have several important responsibilities. First, they can review and assess the medications prescribed to the patient, ensuring that they are appropriate, safe, and effective for managing the condition. This may include insulin or oral medications used to control BGCs. Pharmacists can also collaborate with healthcare providers to adjust medication dosages based on the patient’s blood sugar monitoring results through Collaborative Drug Therapy Management.18
Additionally, pharmacists offer medication counseling to patients, providing comprehensive education about their prescribed medications. This includes guidance on administering insulin injections correctly, using glucose monitoring devices, and understanding potential side effects and drug interactions.19 Pharmacists are accessible to respond to any inquiries or issues the patient may have about her medications.
Moreover, patient education is a key aspect of a pharmacist’s role in managing GDM. Pharmacists can educate patients about the condition itself, including its causes, risk factors, and long-term implications.20 They help patients understand the importance of effective condition management and its potential impact on both maternal and fetal health. Additionally, they provide information on self-care measures, such as foot care, stress management, and the importance of regular prenatal and postpartum check-ups.
BGM is a vital part of managing GDM, and pharmacists play a significant role in this area.21 They educate patients on the importance of regular monitoring and proper use of glucose meters. Pharmacists provide information on optimal BGC ranges, assist patients in understanding their results, and provide advice on appropriate steps based on their readings. They may also recommend suitable glucose monitoring devices and provide instructions on device maintenance. Simultaneously, lifestyle modifications are essential for managing GDM, and pharmacists offer valuable guidance in this regard. They provide advice on maintaining a healthy weight, following a nutritious diet, and engaging in regular exercise.
Conclusion
The rising prevalence of GDM presents a significant challenge that requires vigilant monitoring and effective management. Its intricate pathophysiology, involving hormonal, metabolic, and placental factors, emphasizes the importance of early screening through recognition of risk factors such as elevated BMI, sedentary lifestyle, prediabetes, and family history. Timely identification allows for appropriate treatment, with insulin being the most preferred option, followed by metformin and glyburide with careful consideration.22 Regular BGM and lifestyle changes are essential components of GDM management. Postpartum care is crucial in evaluating glucose metabolism and reducing the risk of T2D development. The involvement of pharmacists in counseling and patient education plays a vital role in supporting better outcomes and reinforcing the necessity of a comprehensive healthcare approach to effectively manage GDM for healthier pregnancies and lives.
REFERENCES
1. CDC. Gestational diabetes. December 30, 2022. www.cdc.gov/diabetes/basics/gestational.html. Accessed August 18, 2023.
2. McIntyre HD, Kapur A, Divakar H, Hod M. Gestational diabetes mellitus—innovative approach to prediction, diagnosis, management, and prevention of future NCD—mother and offspring. Front Endocrinol (Lausanne). 2020;11:614533.
3. Kampmann U, Knorr S, Fuglsang J, Ovesen P. Determinants of maternal insulin resistance during pregnancy: an updated overview. J Diabetes Res. 2019;2019:5320156.
4. National Library of Medicine. Type 1 diabetes: MedlinePlus. February 10, 2023. www.medlineplus.gov/ency/article/000305.htm. Accessed August 18, 2023.
5. Amiri FN, Faramarzi M, Bakhtiari A, Omidvar S. Risk factors for gestational diabetes mellitus: a case-control study. Am J Lifestyle Med. 2018;15(2):184-190.
6. Egan AM, Enninga EAL, Alrahmani L, et al. Recurrent gestational diabetes mellitus: a narrative review and single-center experience. J Clin Med. 2021;10(4):569.
7. Miller C, Lim E. The risk of diabetes after giving birth to a macrosomic infant: data from the NHANES cohort. Matern Health Neonatol Perinatol. 2021;7(1):12.
8. ElSayed NA, Aleppo G, Aroda VR, et al. 2. Classification and diagnosis of diabetes: Standards of Care in Diabetes—2023. Diabetes Care. 2023;46(Suppl 1):s19-S40.
9. American Diabetes Association. Gestational diabetes: gestational diabetes and a healthy baby? Yes. www.diabetes.org/diabetes/gestational-diabetes. Accessed August 18, 2023.
10. American Diabetes Association Professional Practice Committee. 5. Facilitating behavior change and well-being to improve health outcomes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(Suppl 1):s60-s82.
11. Ferrari N, Joisten C. Impact of physical activity on course and outcome of pregnancy from pre- to postnatal. Eur J Clin Nutr. 2021;75(12):1698-1709.
12. Lende M, Rijhsinghani A. Gestational diabetes: overview with emphasis on medical management. Int J Environ Res Public Health. 2020;17(24):9573.
13. Mukherjee SM, Dawson A. Diabetes: how to manage gestational diabetes mellitus. Drugs Context. 2022;11:2021-9-12.
14. Polsky S, Garcetti R, Pyle L, et al. Continuous glucose monitor use with and without remote monitoring in pregnant women with type 1 diabetes: a pilot study. PLoS One. 2020;15(4):e0230476.
15. Priya G, Kalra S. Metformin in the management of diabetes during pregnancy and lactation. Drugs Context. 2018;7:212523.
16. American Diabetes Association Professional Practice Committee. 15. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(Suppl 1):s232-s243.
17. American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2020. Diabetes Care. 2020;43(Suppl 1):s183-s192.
18. Orabone AW, Do V, Cohen E. Pharmacist-managed diabetes programs: improving treatment adherence and patient outcomes. Diabetes Metab Syndr Obes. 2022;15:1911-1923.
19. CDC. Infection safety: infection prevention during blood glucose monitoring and insulin administration. March 2, 2011. www.cdc.gov/injectionsafety/blood-glucose-monitoring.html. Accessed August 18, 2023.
20. Gershman J. Pharmacists are key allies in reducing risk of diabetes, cardiovascular disease. Drug Topics. 2021;165(2).
21. Weinstock RS, Aleppo G, Bailey TS, et al. The role of blood glucose monitoring in diabetes management. ADA Clin Compendia. 2020;2020(3).
22. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19(11):3342.
23. Minschart C, Beunen K, Benhalima K. An update on screening strategies for gestational diabetes mellitus: a narrative review. Diabetes Metab Syndr Obes. 2021;14:3047-3076.
24. Chatzakis C, Cavoretto P, Sotiriadis A. Gestational diabetes mellitus pharmacological prevention and treatment. Curr Pharm Des. 2021;27(36):3833-3840.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





