US Pharm. 2020;45(5):15-19.
ABSTRACT: Treatment-resistant depression (TRD) is a growing area of discussion among researchers and medical professionals. Varying resistance levels among patients with TRD pose difficulties in determining an effective treatment for individual patients. Currently, only a few treatment options are approved with an indication for TRD; therefore, current treatment recommendations include restructured oral therapy regimens, psychotherapy, and other options. The alternative of continuous switching and/or combining of antidepressants requires extensive patient education and counseling on proper administration and potential side effects. The use of guidelines and individual patient response are key factors in finding an optimal treatment approach and effectively managing TRD.
Depression can transpire at any time of life because of various causative factors, regardless of individual characteristics such as age, background, and sex. Although there are no definitive causes, patients with a first-degree relative who has a diagnosis of major depressive disorder (MDD) are two to three times more likely to be diagnosed as well.1 MDD is a common mental-health condition that alters a person’s quality of life, with severe symptoms adversely affecting thoughts, feelings, and interest in usual activities, such as eating or working. A clinical diagnosis of depression requires that symptoms persist daily for at least 2 weeks. There are several categories of depression, including persistent, postpartum, psychotic, seasonal, and resistant depression.2 It is estimated that nearly 25% of people will have an inadequate response to depression therapy, meaning that they are treatment-resistant.3
Diagnosis
To aid in distinguishing between and diagnosing different types of depression, psychiatrists developed the Hamilton Rating Scale for Depression (HAM-D). HAM-D is a 21-item symptom assessment that is used to determine the severity and subtype of the patient’s depression. A widely growing subtype is treatment-resistant depression (TRD).4 Although a unanimous definition has not been reached, current guidelines and research characterize TRD as an inadequate response to two or more antidepressants from separate classes within 4 to 6 weeks after the target dose has been reached.5
The causes of TRD vary, and individual biological characteristics are likely involved. These characteristics, accompanied by the heterogeneity of depression itself, result in TRD.6 Researchers are studying what factors lead to an inadequate response to certain antidepressants, but to date, individual patient characteristics, symptoms, course, and combined comorbidities are considered key factors in TRD development.5 Risk factors that could lead to TRD and possibly alter the efficacy of TRD treatment include the severity of the patient’s depression and the presence of comorbid medical conditions such as diabetes, cancer, chronic pain, and coronary artery disease.7 Both TRD and treatment-responsive depression involve the same broad range of symptoms, but the distinguishing features in patients who experience one form versus the other remain to be clarified.
Treatment Overview
Treatment for depression is not one-size-fits-all.6 Recent research has offered many suggestions for managing the symptoms of TRD, but most findings are mainly empirical, and providers should take a rational approach to initiating treatment methods.8 SACO, a mnemonic developed to aid in the selection of treatment options, aligns with current guidelines’ recommendations of Switching therapies, Augmentation, Combination of antidepressant classes, and Optimization as appropriate approaches for managing TRD.9 Other options include using genetic testing to determine whether genomic variations will affect a patient’s ability to tolerate a certain medication and using medications with off-label antidepressant indications. The complexity of treatment for TRD reflects the diverse nature of the disorder, and the provider should be careful to make an accurate diagnosis before treatment.8 Studies have not shown one treatment approach to be superior to another, and treatment should be based on the individual patient’s disease state. Because of this, TRD treatment will likely be an extensive trial-and-error process. In addition to monitoring for efficacy, it is imperative that the provider monitor for adherence before deeming a treatment approach inadequate; this is because nonadherence could also be a potential link to resistance.9
Nonadherence during treatment for TRD could be due to a variety of factors, the most prominent ones being cost and side effects. Medical costs are nearly 70% higher for patients with TRD than for those without, resulting from workdays missed because of depressive episodes, physician or hospital visits, and medication costs.10,11
When therapy is being initiated, it is reasonable to start a patient on the lowest available dose to determine patient response and then titrate (every 2-4 weeks) to the usual dosing range (TABLE 1) if necessary.1,7,12-15 If a patient’s response to initial conventional dosing is minimal, it is appropriate to increase the dose and reassess prior to switching or augmenting (TABLE 2).16,17 In contrast, if the patient has no response to initial therapy, switching to an alternative agent or class, as suggested in the guidelines, may be beneficial.1
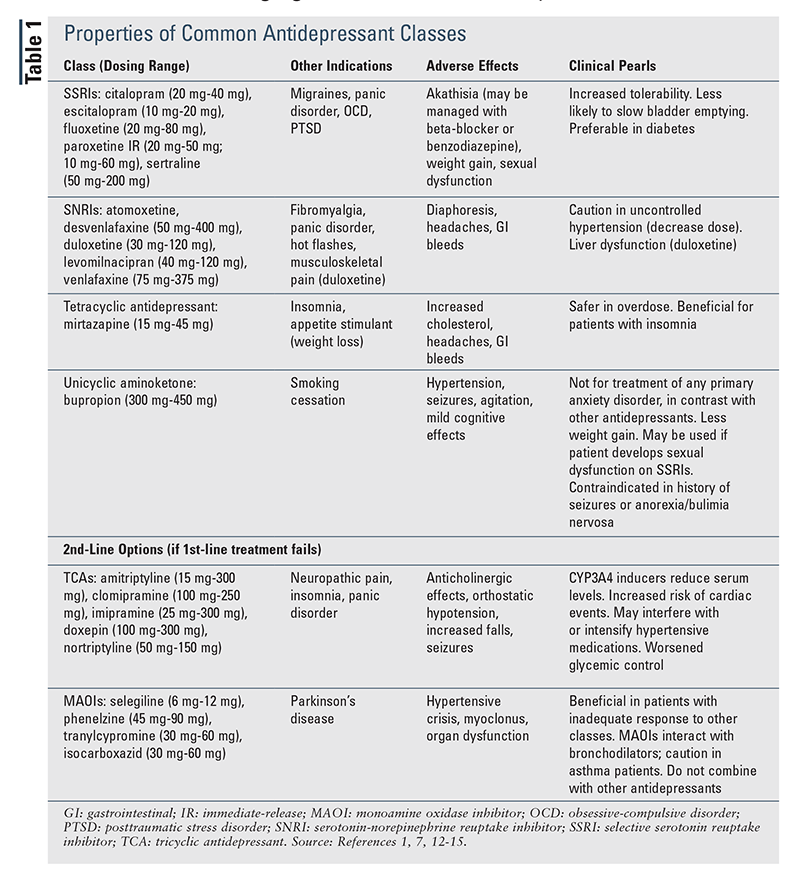
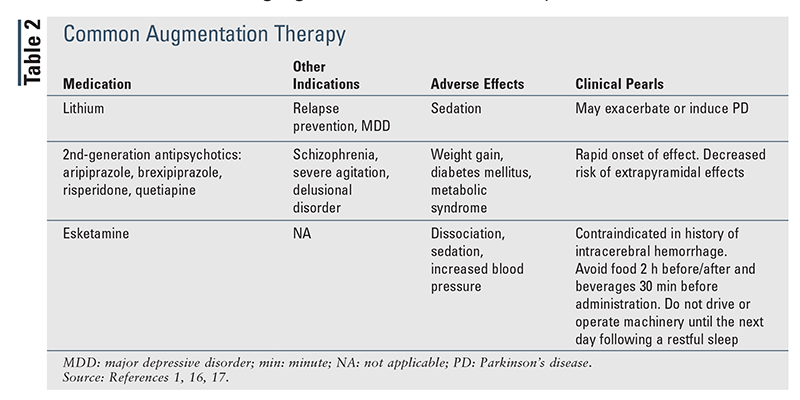
Guideline-Directed Treatment Approaches
Determining the optimal treatment for patients with TRD involves ongoing discussion of many treatment methods. Trials of different medications may be required to achieve the desired treatment outcome for the individual patient. Many guidelines have delineated a variety of methods to optimize a patient’s treatment regimen, including the 2010 American Psychiatric Association (APA) guidelines, the 2016 Canadian guidelines, the Department of Veterans Affairs and Department of Defense (VA/DoD) guidelines, and the 2017-2018 Florida Best Practice guidelines. Given the wide range of treatment approaches available, patients will benefit most from an individualized approach, as the level of resistance is different for each patient.
APA Guidelines (2010): The 2010 APA guidelines include the various treatment strategies outlined in the SACO approach and also provide significant evidence regarding the benefits of switching to alternative medication classes (TABLE 3).1 The guidelines recommend the use of psychotherapy (cognitive-behavioral therapy [CBT], interpersonal psychotherapy [IPT]) or monotherapy with a common antidepressant for first-line therapy. Treatment response should be monitored initially, at 4 to 8 weeks, and throughout treatment. If a patient experiences severe or life-threatening symptoms with current therapy, consideration should be given to electroconvulsive therapy (ECT), dose reduction, treatment augmentation, treatment of individual side effects, or an alternative medication (tricyclic antidepressant [TCA], monoamine oxidase inhibitor [MAOI], lithium, thyroid therapy, 2nd-generation antipsychotic).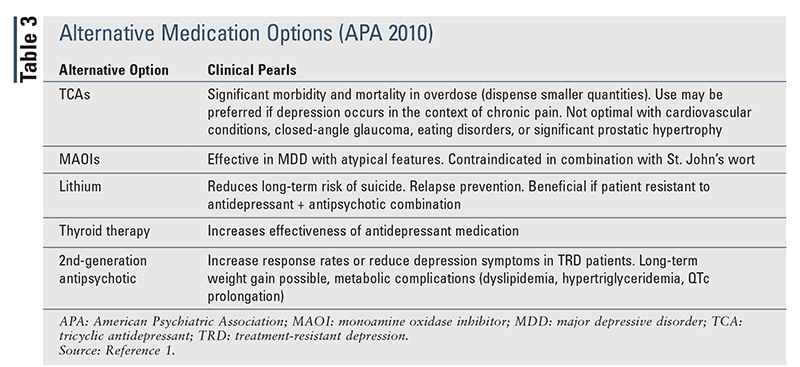
Canadian Guidelines (2016): The Canadian guidelines (FIGURE 1) provide suggestions for alternative medications based on their ranking for line of treatment.5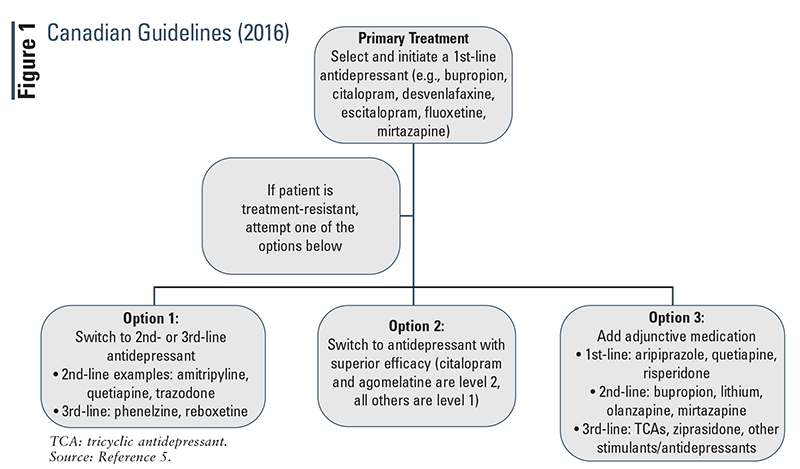
2016 VA/DoD Guidelines: The Va/DoD guidelines recommend psychotherapy (CBT, IPT, problem-solving therapy) and appropriate monotherapy as initial treatment in patients with MDD. If the patient has an inadequate response to initial treatment, olanzapine plus fluoxetine is suggested. Olanzapine monotherapy is not indicated for TRD treatment; it should used only in combination. This was the only guideline assessed that specified the olanzapine-plus-fluoxetine combination as a viable option for patients with TRD. If an inadequate response persists following two pharmacotherapy trials, it is appropriate to switch the patient to an MAOI or TCA. Following initiation or any dose changes, patients should be monitored at least monthly until remission.15
Florida Best Practice Psychotherapeutic Medication Guidelines for Adults (2017-2018): This guideline uses levels to distinguish different treatment stages (FIGURE 2). If a patient has an inadequate response on one level, move to the next. Diagnosis should be evaluated following an inadequate response to levels 1 and 2.18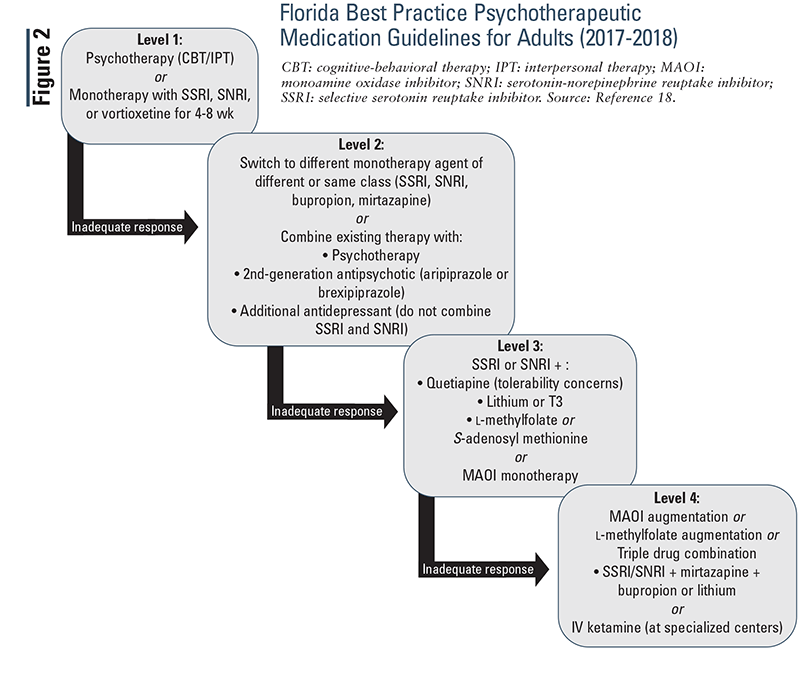
Alternative Approaches
Additional treatment options listed in the guidelines are ECT and vagus nerve stimulation (VNS). All of the guidelines advise that ECT be reserved for use after a patient has an inadequate response (or intolerance) to several trials of antidepressant classes. The APA suggests ECT as a first-line option for patients who prefer it or those with psychotic symptoms or a positive response to psychotherapy in the past. VNS is suggested as a last-line option, and it is advised against in the VA/DoD guidelines. Transcranial magnetic stimulation, the only FDA-approved somatic therapy, is suggested in all guidelines (except the Canadian guidelines) as a viable treatment option for TRD if pharmacotherapy trials fail.1,5,15
FDA-Approved Drugs
Esketamine (Spravato), a ketamine isomer, is the newest FDA-approved treatment option for TRD and is indicated in conjunction with an oral antidepressant. The intranasal formulation allows it to bypass the oral-bioavailability issues seen with ketamine and enables it to reach the brain faster, resulting in a quicker onset of antidepressant effects.19 Symbyax (olanzapine-fluoxetine) is the only other FDA-approved (2009) pharmacotherapy option for TRD.20
The Pharmacist’s Role
Pharmacists play a critical role in the treatment of TRD. They can assist with medication selection and appropriate dosage, and they also can help identify possible drug interactions that may warrant alternative medications.Nonadherence due to lack of motivation or excessive side effects is a big problem with TRD treatment.9 Pharmacists can help ensure medication adherence by counseling patients on onset of action, possible side effects, and the importance of adherence. It is essential that the pharmacist relay any necessary information to the prescriber so that the patient can receive optimal treatment benefits.
Conclusion
Finding an effective treatment regimen for a patient with TRD is largely trial-and-error. There is no superiority among the various treatment approaches, including dose reduction, optimization, switching, and augmentation, allowing more individualized regimens. Patients should be monitored closely, and transparency regarding any concerns should be encouraged. Treatment is successful when provider, patient, and pharmacist work cohesively to reach the goal of symptom management.
REFERENCES
1. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice Guideline for the Treatment of Patients With Major Depressive Disorder. 3rd ed. Washington, DC: American Psychiatric Association; 2010.
2. National Institute of Mental Health. Depression. www.nimh.nih.gov/health/topics/depression/index.shtml. Accessed January 10, 2020.
3. Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin N Am. 1996;19:179-200.
4. Rohan KJ, Rough JN, Evans M, et al. A protocol for the Hamilton Rating Scale for Depression: item scoring rules, rater training, and outcome accuracy with data on its application in a clinical trial. J Affect Disord. 2016;200:111-118.
5. Kennedy SH, Lam RW, McIntyre RS, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry. 2016;61:540-560.
6. Akil H, Gordon J, Hen R, et al. Treatment resistant depression: a multi-scale, systems biology approach. Neurosci Biobehav Rev. 2018;84:272-288.
7. Trevino K, McClintock SM, McDonald Fischer N, et al. Defining treatment-resistant depression: a comprehensive review of the literature. Ann Clin Psychiatry. 2014;26:222-232.
8. Pandarakalam JP. Challenges of treatment-resistant depression. Psychiatr Danub. 2018;30:273-284.
9. Ionescu DF, Rosenbaum JF, Alpert JE. Pharmacological approaches to the challenge of treatment-resistant depression. Dialogues Clin Neurosci. 2015;17:111-126.
10. Mrazek DA, Hornberger JC, Altar CA, Degtiar I. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996-2013. Psychiatr Serv. 2014;65:977-987.
11. Pilon D, Joshi K, Sheehan JJ, et al. Burden of treatment-resistant depression in Medicare: a retrospective claims database analysis. PLoS One. 2019;14:e0223255.
12. Dezsi L, Vecsei L. Monoamine oxidase B inhibitors in Parkinson’s disease. CNS Neuro Disord Drug Targets. 2017;16:425-439.
13. Fekadu A, Wooderson S, Donaldson C, et al. A multidimensional tool to quantify treatment resistance in depression: the Maudsley staging method. J Clin Psychiatry. 2009;70:177-184.
14. Reid R, Abramson BL, Blake J, et al. Managing menopause. J Obstet Gynaecol Can. 2014;36:830-833.
15. Management of Major Depressive Disorder Working Group. VA/DoD Clinical Practice Guideline for the management of major depressive disorder. Version 3.0; 2016. www.healthquality.va.gov/guidelines/MH/mdd/VADoDMDDCPGFINAL82916.pdf. Accessed January 20, 2020.
16. Chokhawala K, Stevens L. Antipsychotic medications. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2020.
17. Spravato (esketamine) package insert. Titusville, NJ: Janssen Pharmaceuticals, Inc; February 2020.
18. McIntyre RS, Suppes T, Tandon R, Ostacher M. Florida Best Practice Psychotherapeutic Medication Guidelines for adults with major depressive disorder. J Clin Psychiatry. 2017;78:703-713.
19. Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75:139-148.
20. Bobo WV, Shelton RC. Efficacy, safety and tolerability of Symbyax for acute-phase management of treatment-resistant depression. Expert Rev Neurother. 2010;10:651-670.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






