US Pharm. 2019;44(7):8-13.
Since their introduction, vaccines have made a significant impact on human lives and public health. The success of vaccines in the United States is due in large part to the Vaccines for Children program (VFC), which launched in 1994 as a direct response to the resurgence of measles nationally. The CDC estimates that vaccination of children born between 1994 and 2018 will prevent 419 million illnesses, help avoid 936,000 deaths, and save nearly $1.9 trillion in total societal costs.1
The VFC program is a federally funded program that provides vaccines at no cost to children who might not otherwise be vaccinated because of inability to pay, giving all children a better chance of getting their recommended vaccines. Despite the availability of the measles vaccine—specifically the measles, mumps and rubella (MMR) vaccine—and the VFC program, there are still many children across the nation left unvaccinated.2
Vaccines work by imitating an infection, thereby helping develop immunity. This “infection” is very unlikely to cause illness, but it boosts the immune system’s production of T-lymphocytes and antibodies. Once this “infection” disappears, the body is left with a supply of “memory” T-lymphocytes and B-lymphocytes that will remember how to fight that disease in the future. By working with the body’s natural defenses to develop immunity, vaccines prevent diseases that can be dangerous or deadly.3
Besides protecting the individual who is vaccinated, other people are safer as well through a concept known as herd immunity. Germs can travel quickly throughout a community and lead to an outbreak when enough people get sick. However, if a majority of a population is vaccinated, germs cannot travel as easily and the entire community is less likely to get sick. Herd immunity only applies to communicable diseases and requires a large proportion of the population to be vaccinated. The threshold, or minimum percentage of immune individuals needed to establish herd immunity, is dependent upon how infectious the disease is. For example, it is estimated that 93% to 95% of a population needs to be vaccinated to establish herd immunity against measles, whereas the threshold for polio is 80% to 86%. This concept of herd immunity is especially beneficial for the most vulnerable members of our communities, including infants, pregnant women, and those who are not able to receive certain vaccinations due to contraindications. Even if a person gets sick, there is less chance of an outbreak because it is more difficult for the disease to spread. Eventually, the disease becomes rare and may be eradicated altogether.4-6
Measles
The first component of the MMR vaccine prevents measles, otherwise known as rubeola, a highly contagious viral infectious disease that can be traced back as far as the 7th century. Before a vaccine was available, infection with measles was endemic, affecting most children; more than 50% of children had measles by age 6, and more than 90% of children had measles and were immune by age 15. The highest incidence of measles was among children aged 5 to 9 years, accounting for more than 50% of cases. Measles is still a common and often fatal disease in developing countries. The World Health Organization estimates there were 145,700 deaths globally from measles in 2013.7
The measles virus is a paramyxovirus. Two membrane-envelope proteins are important in its pathogenesis. They are the F (fusion) protein, which is responsible for fusion of the virus and host-cell membranes, viral penetration, and hemolysis, and the H (hemagglutinin) protein, which is responsible for adsorption of the virus to cells. There is only one antigenic type of measles virus, and although studies have documented changes in the H glycoprotein, no change in vaccine efficacy has been observed.
Measles is a systemic infection and a respiratory virus transmitted primarily person-to-person via large respiratory droplets, either airborne or through direct contact. Respiratory droplets that people sneeze, cough, drip, or exhale are loaded with infectious particles and travel only short distances before settling. The virus itself is rapidly inactivated; it can survive in the air or on objects for less than 2 hours.7
Measles is a highly infectious disease; an estimated 90% of nonimmune persons who are exposed to the virus will develop the disease. The average incubation period from exposure to early symptoms is approximately 10 to 12 days, with 14 days from exposure to rash onset. Infected patients are contagious from 4 days before through 4 days after rash development. Prodromal symptoms include a cough, runny nose, conjunctivitis, and fever that usually peaks around 103°F–105°F. The measles rash starts at the head and spreads downward to the rest of the body. It lasts for up to 7 days and fades in the order of appearance. Other symptoms may include abdominal pain, diarrhea, and generalized lymphadenopathy.7-9
Mumps and Rubella
The second component of the MMR vaccine prevents mumps, an acute viral illness caused by a paramyxovirus; the most common symptom associated with mumps is parotitis. Vaccination reduced the number of reported mumps cases from 152,000 in 1968 to 338 in 2000.10,11
The currently used mumps vaccine was licensed in the U.S. in 1967. Initially, one dose of the mumps vaccine was recommended for all children at any age after 12 months. In 1989, children began receiving 2 doses of mumps vaccine because of a 2-dose measles vaccination policy using the combined MMR vaccine.10
The third component of the MMR vaccine prevents rubella, a viral illness classified as a togavirus and characterized by a mild, maculopapular rash.12,13
Since the introduction of the live attenuated rubella vaccines in the U.S., the number of reported cases of rubella in the U.S. has declined to a median of 11 cases annually, reported during the period of 2005–2011.13
History of Measles and the MMR Vaccine
In 1912, measles was established as a nationally notifiable disease in the U.S., requiring healthcare providers and laboratories to report all diagnosed cases. Prior to the availability of a vaccine, almost all children contracted measles by the time they were aged 15 years. Measles infected an estimated 3 million to 4 million people with up to 500 reported deaths annually.14
The CDC set a goal in 1978 to eliminate measles from the U.S. by 1982. Although this goal was not met, the use of the measles vaccine drastically reduced disease rates. By 1981, the number of reported measles cases was 80% less than the previous year. In 1989, measles outbreaks among vaccinated school-age children prompted the recommendation of a second dose of MMR vaccine for all children. This led to improvements in first-dose MMR vaccine coverage, and with further strides in the nation’s vaccination program, measles and rubella were eventually declared eliminated from the U.S. in 2000.14
In 1963, the first measles vaccines, both an inactivated and a live attenuated vaccine, were licensed for use in the U.S. Today, the only measles vaccine available in the U.S. is a live attenuated vaccine. The measles vaccine is administered in combination with the mumps and rubella vaccines as MMR. In 2016, 91.1% of children aged 19 months to 35 months received MMR. This percentage of patients vaccinated with MMR has remained consistently between 90% to 92%; almost 10% of the U.S. population remains unvaccinated.7,15
Efficacy of MMR
The MMR vaccine is very safe and effective. It is given as a 2-dose series. The first dose of MMR should be administered anytime between ages 12 months and 15 months, with the second dose administered between ages 4 years and 6 years. Adults should have at least one dose of MMR vaccine if they do not have any evidence of immunity. Two doses of the vaccine are approximately 97% effective at preventing measles, while one dose is about 93% effective. Measles antibodies develop in approximately 95% of children vaccinated at 12 months of age and 98% of children vaccinated at 15 months of age. Approximately 2% to 5% of children who receive only one dose of MMR vaccine fail to respond to it; however, most persons who fail to respond to the first dose will respond to a second dose.7,16
Safety of MMR
The MMR vaccine has a long record of safety, and serious adverse reactions from MMR are rare. To date, there is no convincing evidence to support the relationship of any vaccine causing autism or autism-spectrum disorders. Adverse reactions usually occur within 5 to 12 days after vaccination and may include fever and mild rash. Up to 25% of adult women may experience arthralgias and other joint symptoms, which are believed to be related to the rubella component. Allergic reactions including rash, pruritus, and purpura have been temporally associated with MMR vaccination, but these are uncommon and usually mild and of brief duration. Allergic reactions happen less than one time per one million vaccine doses.7,17
Contraindications and Precautions
Contraindications for the MMR vaccine include a history of anaphylactic reactions to neomycin, a history of a severe allergic reaction to any component of the vaccine, pregnancy, receipt of antibody-containing blood products, and immunosuppression. Pregnancy should be avoided for 4 weeks following the MMR vaccine. Close contact with a pregnant woman is not a contraindication. Measles can be severe in patients infected with HIV; as long as an individual infected with HIV does not have any evidence of current severe immunosuppression, MMR is recommended for all persons who are aged 12 months or older.7
Recent Measles Outbreaks
Measles has remained endemic in other parts of the world where the vaccine is not easily accessible. This includes countries within Europe, Asia, the Pacific Islands, and Africa. In 2017, there were 110,000 measles deaths globally, mostly among children younger than age 5 years. Despite the large number of deaths caused by measles every year, measles vaccination resulted in an 80% drop in measles deaths, preventing an estimated 21.1 million deaths between 2000 and 2017.18
Despite having been declared eliminated in 2000 within the U.S., there have been a number of outbreaks reported over the past 10 years, primarily among unvaccinated populations. Many cases of measles occur because of an increase in travelers who contract measles abroad and bring it into the U.S. The further spread of measles within communities is attributed to poor vaccination coverage. The U.S. is currently experiencing the worst measles outbreak in a quarter-century. There has been a recent increase in the number of measles outbreaks in communities across the country, including New York, New Jersey, Washington, California, and Michigan. As of June 6, 2019, there have been 1,022 confirmed measles cases in 28 states. This is the greatest number of cases reported in the U.S. since 1992, when 2,126 cases were reported.19
Stopping the measles outbreak through vaccination is a top priority for the CDC. However, a significant factor contributing to these outbreaks is misinformation about the safety and efficacy of the MMR vaccine. The agency is urging local leaders to provide accurate, scientifically based information to counter misinformation. As accessible healthcare professionals, pharmacists should be promoting the benefits and safety of this vaccine. In addition, some municipalities are making efforts to decrease mandatory vaccine exemptions and limiting access to public spaces for unvaccinated individuals.20
Pharmacist’s Role
Pharmacists are in a unique position to educate patients and parents about the importance of vaccination and dispel the myths surrounding their administration, in particular the MMR vaccine. Pharmacists can answer questions, address concerns, and provide recommendations regarding vaccinations. Vaccination screenings should be incorporated with all patient interactions, along with strong recommendations for necessary vaccines. Pharmacists can raise awareness and engage patients by providing accurate and consistent messages about the safety and appropriate timing of vaccines.
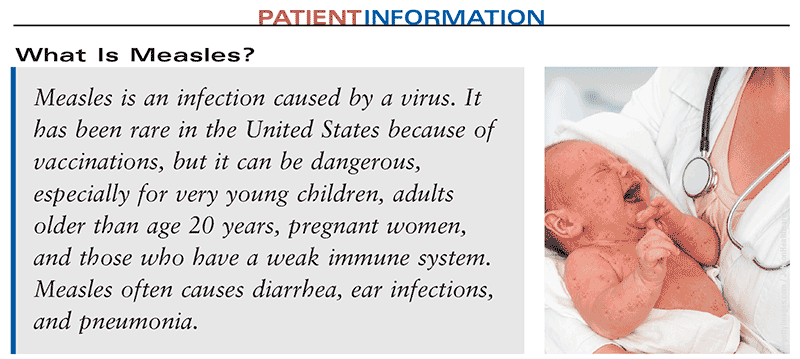
What are the signs and symptoms?
Symptoms will begin to appear 7 to 14 days after exposure to the virus. Symptoms include high fever, runny nose, cough, and red, watery eyes. Children may also have small red spots with blue-white centers inside their mouths before the rash starts. The measles rash does not appear until 3 to 5 days after the first symptoms and lasts about 5 to 6 days. It usually starts on the head and moves downward.
How can someone get measles?
Measles is very contagious and spreads when a person infected with the measles virus breathes, coughs, or sneezes. Measles can be transmitted by being in the airspace where an infected person coughed or sneezed up to 2 hours before. Infected people can spread the disease from 4 days before until 4 days after the rash develops. Up to 90% of people who have not been vaccinated for measles will get the disease if they are exposed to the measles virus.
How is measles treated?
There is no specific treatment for measles. To help with symptoms, get plenty of rest, drink plenty of fluids, and take something to help reduce the fever, such as acetaminophen or ibuprofen.
Can measles be prevented?
This best way to prevent measles is to receive a measles vaccine. For most people, measles protection is provided as part of the measles, mumps, and rubella (MMR) vaccine. There have been fewer cases in the United States because of this vaccine. It is given in two doses, usually when a child is between age 12 months to 15 months and again when they are aged 4 years to 6 years. After the first dose, up to 95% of people will have immunity against measles.
Who should receive the MMR vaccine?
The CDC recommends that all children get two doses of MMR vaccine, starting with the first dose at age 12 months through 15 months, and the second dose at age 4 years through 6 years. Adults who do not have evidence of immunity should get at least one dose of MMR vaccine. People aged 6 months and older who will be traveling internationally should be protected against measles as well.
Does the MMR vaccine have side effects?
Most children do not have any side effects from the shot. Those that do occur are usually mild in severity and duration, such as a fever, rash, and soreness. There could also be swelling at the injection site or some pain and stiffness in the joints (mostly in adult women). A very small number of people may experience more serious side effects, including a high fever that could induce a seizure.
Where can I get more information?
You can speak with your pharmacist, doctor, or other healthcare provider. You can also contact your local or state health department or call the CDC at 1-800-232-4636 (1-800-CDC-INFO) or visit the CDC’s website at www.cdc.gov/vaccines.
REFERENCES
1. CDC. Vaccines for children program (VFC). Protecting America’s children every day. Published April 2, 2019. www.cdc.gov/vaccines/programs/vfc/protecting-children.html. Accessed June 10, 2019.
2. CDC. Vaccines for children program (VFC). About VFC. Published March 7, 2019. www.cdc.gov/vaccines/programs/vfc/about/index.html. Accessed June 10, 2019.
3. CDC. Understanding how vaccines work. Published March 13, 2019. www.cdc.gov/vaccines/hcp/conversations/understanding-vacc-work.html. Accessed June 12, 2019.
4. Smith PG. Concepts of herd protection and immunity. Procedia in Vaccinology. 2010;2(2):134-139.
5. Mallory ML, Lindesmith LC, Baric RS. Vaccination-induced herd immunity: successes and challenges. J Allergy Clin Immunol. 2018;142(1):64-66.
6. Orenstein WA, Strebel PM, Papania M, et al. Measles eradication: is it in our future? Am J Public Health. 2000;90(10):1521-1525.
7. CDC. The Pinkbook. Epidemiology of vaccine preventable diseases. Measles. Published March 29, 2019. www.cdc.gov/vaccines/pubs/pinkbook/meas.html. Accessed June 10, 2019.
8. McClean HQ, Fiebelkorn AP, Temte JL, Wallace GS; CDC. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62(RR-04):1-34.
9. Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis. 2004;189(Suppl 1):S4-S16.
10. CDC. Manual for the surveillance of vaccine-preventable diseases. Mumps. www.cdc.gov/vaccines/pubs/surv-manual/chpt09-mumps.html. Published March 29, 2019. Accessed June 10, 2019.
11. CDC. The Pinkbook. Epidemiology of vaccine preventable diseases. Mumps. Published March 29, 2019. www.cdc.gov/vaccines/pubs/pinkbook/mumps.html. Accessed June 10, 2019.
12. CDC. The Pinkbook. Epidemiology of vaccine preventable diseases. Rubella. Published March 29, 2019. www.cdc.gov/vaccines/pubs/pinkbook/rubella.html. Accessed June 12, 2019.
13. CDC. Manual for the surveillance of vaccine-preventable diseases. Rubella. Published March 29, 2019. www.cdc.gov/vaccines/pubs/surv-manual/chpt14-rubella.html. Accessed June 12, 2019.
14. CDC. Measles. Measles history. Published February 25, 2019. www.cdc.gov/measles/about/history.html. Accessed June 12, 2019.
15. CDC. FastStats. Immunization. Published February 26, 2019. www.cdc.gov/nchs/fastats/immunize.htm. Accessed June 12, 2019.
16. CDC. Vaccines and preventable diseases. Measles vaccination. Published June 10, 2019. www.cdc.gov/vaccines/vpd/measles/index.html. Accessed June 12, 2019.
17. CDC. Provider resources for conversations with parents. Understanding vaccines and vaccine safety. Published May 28, 2019. www.cdc.gov/vaccines/hcp/conversations/provider-resources-safetysheets.html. Accessed June 12, 2019.
18. World Health Organization. Measles. www.who.int/news-room/fact-sheets/detail/measles. Accessed June 12, 2019.
19. CDC. Measles cases and outbreaks. Measles cases in 2019. www.cdc.gov/measles/cases-outbreaks.html. Published June 10, 2019. Accessed June 12, 2019.
20. CDC Washington. U.S. public health response to the measles outbreak. Testimony of Nancy Messonier, M.D., to the House Energy and Commerce Subcommittee on Oversight and Investigations. February 27, 2019. www.cdc.gov/washington/testimony/2019/t20190227.htm. Accessed June 13, 2019.
To comment on this article, contact rdavidson@uspharmacist.com.