US Pharm. 2016;41(1):45-49.
ABSTRACT: Medical cannabis (marijuana) is reemerging as a treatment option for many medical conditions in the United States. The cannabis plant contains chemicals that are both psychoactive (delta-9-tetrahydrocannabinol [THC]) as well as nonpsychoactive (cannabidiol [CBD]). Its components are being successfully used to lessen seizure activity, treat pain and muscle spasticity, provide neuroprotection, reverse neuronal damage, and reduce symptoms of Parkinson’s disease, amyotrophic lateral sclerosis (ALS), and several other neurologic conditions via important effects on the endocannabinoid system. Despite federal restrictions, twenty-four states and Washington, DC, have passed laws allowing the recommendation and use of medical cannabis in selected patients. Legislators, industry, and leaders in healthcare professions should be encouraged to increase efforts to remove barriers to research.
In opposition to and without the support of federal law, individual states have been passing laws through legislative action or citizen initiative that allow physicians to recommend medical cannabis to selected patients.1 The medical use and regulation of cannabis have a long and interesting history, both nationally and internationally. While the terms medical marijuana and cannabis are often used interchangeably, practitioners who recommend it prefer the scientific term cannabis, as it is used in historical documents. The Marihuana Tax Act of 1937 basically introduced the controversial word “marijuana” into federal policy, during a time of political and racial unrest.2 The law, in effect, made the cultivation, sale, and use of the plant an impossibility for prescribers and patients.3
State vs. Federal Law
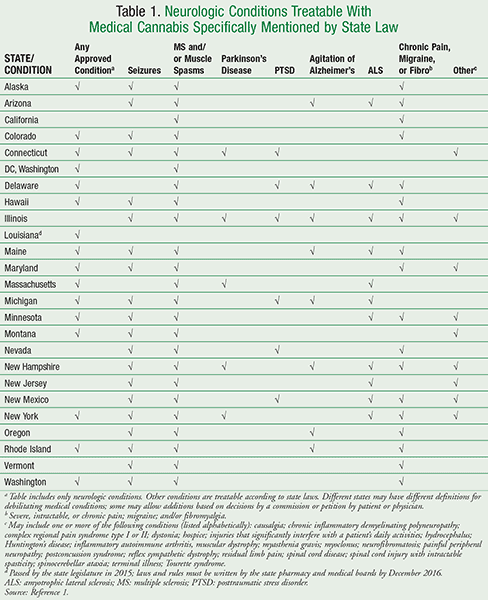
Medical professionals face a variety of challenges if and when the use of cannabis is considered. Over objections by the American Medical Association and the Shafer Commission, in the mid 1970s the U.S. federal government placed cannabis in the Schedule I controlled substance category (C-I), indicating that it has no currently accepted medical use. It has legally remained as marijuana through the present time.2,4 In states where medical marijuana is legal as a C-I controlled substance, federal law still prohibits its prescribing. State laws require that a physician provide documentation that it may be used for a specific patient. The medical conditions for which cannabis may be used vary from state to state (TABLE 1); procedures for recommendations and dispensing also vary.1 Two states, Connecticut and Louisiana, have specified that pharmacists be involved in the dispensing of cannabis.5,6 A difficult application process for the study of cannabis, as well as fear of breaking the law, remain as barriers to performing efficient studies of the cannabis plant and its components, and may prevent open discussion or recommendation of cannabis as a therapeutic alternative.
Other notable issues and challenges that physicians and pharmacists should be aware of include dosing, dispensing, psychoactive and long-term effects of the plant and its components, standardization, availability, and whether or not rigorous evidence exists for safety and efficacy for smoking or ingestion of the plant or its refined products.
Dosing and Safety Considerations
Dosage forms and delivery methods used in case reports and studies vary. Strains of cannabis differ and contain various amounts and ratios of medicinally active components. Raw cannabis is heated to decarboxylate some of the inactive medicinal components to their active forms. Cannabis is often heated and inhaled via vaporizing devices or as rolled cigarettes. Extracts may be placed in oils, tinctures, or other edible vehicles; oils may be placed in capsules or drawn up into individual oral dosing syringes.7-9
The standardization and safety of preparations have rightfully been questioned; practitioners and patients should be able to verify concentration or ingredients. Patients who are stabilized on a particular preparation may find that a product is no longer available from a dispensary or grower. This problem can be circumvented by the realization that cannabis is usually titrated for symptomatic relief and tolerability, and a new strain may be retitrated if needed. Dosing and dosage forms should be discussed and agreed upon by patient and practitioner. Finally, different dosage forms have different pharmaco-kinetic and drug interaction profiles. Bioavailability is affected by its route of administration—inhaled cannabis may be compared to IV administration and is rapidly absorbed with a faster onset of action; ingested cannabis is more slowly absorbed and undergoes first-pass metabolism in the liver. Hepatic metabolism of cannabis makes it susceptible to interactions via the CYP450 enzymes. Practitioners must be educated in this area.7-9
Concerns about psychoactive and long-term effects of medical cannabis have been expressed by many patients and healthcare professionals. The two most studied active components of the cannabis plant include delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). The study and use of CBD in various medical conditions have shown positive results in symptomatic relief and disease progression while seeing a decrease in the unpleasant side effects.10 THC has several unpleasant side effects, including euphoria, tachycardia, and other psychoactive effects. Most are mild and reversible. Drug interactions, mediated by the CYP450 enzymes 3A4, 2D9, and 2C19, have been identified. There are concerning effects on cognitive development in adolescents; however, these are also seen with other agents used for neurologic disease. Some of the cognitive deficits seen in heavy, long-term recreational use might not be applicable to controlled use of medical cannabis.9
The Endocannabinoid System and Phytocannabinoids
Endocannabinoid System: Endogenous cannabinoids are produced by the human body and other animals. Two endogenous cannabinoids that have been identified include anandamide (arachidonoyl ethanolamide) and 2-arachidonoylglycerol (2AG). Unlike some other neurotransmitters (e.g., serotonin, norepinephrine, and acetylcholine), anandamide is not stored in presynaptic vesicles. It is produced in small amounts on demand during inflammatory processes, exhibiting retrograde neurotransmission. Anandamide has a very short half-life before being degraded by the enzymes fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL).11
Phytocannabinoids: Notable chemicals in the cannabis plant include phytocannabinoids and terpenes. Over 100 cannabinoids in the plant have been identified, of which THC and CBD are the most prominent.12 THC is psychoactive and has several known uses in the medical world. Two FDA-approved synthetic drugs containing THC include dronabinol (Marinol) and nabilone (Cesamet), which are used in anorexia, nausea and vomiting associated with chemotherapy, and AIDS.13,14 CBD and the other cannabinoids also have known medical properties, with little or no psychoactivity compared to THC. A combination of CBD and THC, nabiximols (Sativex), is approved in the United Kingdom and several other countries for treatment of spasticity due to multiple sclerosis (discussed in more detail under Multiple Sclerosis).15,16
Terpenes are plant chemicals that confer aroma and taste to plants, which also have medical value, and are part of the plant’s “entourage effect.”17 The plants Cannabis sativa and Cannabis indica have been hybridized to contain different amounts and ratios of THC, CBD, other cannabinoids, and terpenes.18 “Charlotte’s Web” (discussed further under Epilepsy) is an extract from a cannabis strain with a high CBD–low THC ratio, used in the treatment of epilepsy in states where medical cannabis is legal. Higher CBD-THC ratios may reduce some or all of the unwanted psychotropic effects.19 As THC also has desirable medicinal properties, including anticonvulsant effects, it might be worth noting that while the most renowned cases where cannabidiol was used to successfully treat neurologic diseases demonstrate its efficacy, some patients may require a cannabinoid profile where more THC is needed in the ratio.
Target receptors of both endocannabinoids and phytocannabinoids include the CB1 and CB2 receptors, which are located throughout the body. CB1 and CB2 cluster in areas of the central nervous system (CNS) that mediate pain, memory, and other key functions. CB1 receptors are highly concentrated in the brain, whereas CB2 receptors are found primarily in the immune cells.7,11
Neurotoxicity and Neuroprotection: Most neurologic disease is attributed to inflammation and neuronal injuries involving some form of damage caused by glutamate, cytokines, thromboxane, reactive oxygen, and ischemia. Excitotoxicity is a process that is seen in neurodegenerative disorders; amino acid receptors on neurons are stimulated excessively, especially by the amino acid glutamate, which permits the influx of calcium and sodium into the cell. Excessive calcium influx damages cell structures, leading to apoptosis.20,21
Neuroprotection is the slowing of neurodegeneration, which can slow disease progression and cell death. Endocannabinoids are released during neuronal injury to promote healing and homeostasis; phytocannabinoids could extend this mechanism of neuroprotection. Many of the cannabinoids are antioxidants and protective against glutamate, an excitatory neurotransmitter, in cellular and animal models.8,21 Hampson et al found that glutamate toxicity could be reduced by administration of THC and CBD.8,22 Exogenous administration of anandamide and 2AG to rats decreased cytotoxic edema and infarct size.8,23
Use of Cannabis in Neurologic Diseases
Epilepsy: Approximately 30% of epileptic patients on two or more antiepileptic drugs (AEDs) continue to be resistant to treatment, resulting in poor seizure control.24 When treatment failure or side effects from AEDs are no longer acceptable to patients or parents of patients with treatment-resistant epilepsy (TRE), alternatives are needed. Evidence for the use of cannabis in convulsive disorders has long been documented, but rigorous studies comparing cannabinoids to placebo or AEDs have previously been lacking. A growing list of studies found in medical databases has led to the recognition of CBD as an alternative or addition to traditional AEDs for TRE.25 THC and CBD have been shown to have anticonvulsant effects, but the adverse effects of THC are usually undesirable, especially in children. According to Cilio et al, there is “strong evidence for the safety and anticonvulsant properties of CBD.”26
Possible mechanisms involved in anticonvulsive effects include modulation of transient receptor potential (TRP) channel interactions with intracellular calcium and G protein–coupled receptor (GPCR) or voltage-dependent anion-selective channel (VDAC) proteins; reduction of neuronal transmission and excitation; inhibition of adenosine reuptake; and anti-inflammatory effects involving tumor necrosis factor-alpha. Other cannabinoids in the plant may also have anticonvulsant properties; further study is warranted.25
Maa and Figi described a case report of a young patient (Charlotte Figi) with severe epilepsy who was successfully treated with a cannabidiol extract, now known as Charlotte’s Web.19,27,28 The patient began having seizures at 3 months of age and was diagnosed with Dravet syndrome. This condition is characterized by several seizure types that may be lengthy (lasting at least 30 minutes), numerous (>100 per day or month), and difficult to control with traditional AEDs. In addition to the seizures, quality of life is affected by behavioral disturbances and poor language, motor, and developmental skills. Early onset seizures (age <3 years) have been associated with lower IQ scores later in life. Some of these untoward effects are associated with the AEDs themselves, both individually and additively, including extreme sedation, cognitive impairment, gingival hyperplasia, hirsutism, risk of birth defects, liver toxicity, and electrolyte imbalances.19,27,28
In 2012, at 5 years old, Charlotte was experiencing over 300 seizures a week, some lasting several hours.19 Due to these severe circumstances, the use of medical marijuana for the youngest patient in Colorado was approved. To avoid psychotropic adverse effects, a strain was sought that had a high level of CBD and a low level of THC. An extraction was made and given to the child sublingually, who was subsequently seizure free for 7 days. The supply was quickly depleted and no longer available. Growers who had cross bred a high CBD/low THC strain were contacted, who then began providing the needed supply. As of 2014, seizures were decreased from 50 per day to 2 or 3 per month, with improvement in motor and cognitive functions and behavior; she has been weaned from other AEDs. In addition, over 200 patients have been treated with Charlotte’s Web.19 A 1:1 mixture of CBD-THC (Epidiolex) is currently being investigated for use in Dravet syndrome and Lennox-Gastaut syndrome, a severe form of epilepsy that begins in childhood.29
Multiple Sclerosis (MS): Studies have shown that cannabis extract is effective for treatment of symptoms of MS, including muscle spasticity, pain, sleep quality, and bladder dysfunction.30-33 Side effects of traditional muscle relaxants for treatment of these symptoms are often not well-tolerated. A randomized, placebo-controlled, double-blind study published in 2010 involved 337 patients with MS-related spasticity. It showed significant symptom reduction for those who did not find relief with current treatments.34 MS pathology includes inflammatory processes, including infiltration of macrophages and B and T cells; chronic inflammation causes degradation of myelin sheath on nerve fibers, with changes to astrocytes and microglia, leading to muscle spasms, tremors, pain, sleep disturbances, and incontinence. It has been reported that during CNS inflammation, the endocannabinoid system is highly activated; anandamide protects neurons from inflammatory damage.
The CBD-THC extract nabiximols (Sativex), an oral mucosal spray, has been investigated in multiple studies for MS-related spasticity, neuropathic and cancer pain, and bladder dysfunction. It is currently approved in several countries and is in phase III trials in the United States.15,16 Adverse effects are usually mild, transient, and well-tolerated. They include dizziness, mood changes, anxiety, and paranoia and are expected to dissipate upon dose reduction or discontinuation. Because cannabinoids have active metabolites that are excreted in the urine, effects may be enhanced or prolonged in patients with renal or hepatic disease, and these populations should be monitored closely. Confusion, delusions, psychosis, and suicidal ideation have also been reported; the medication should be discontinued immediately if these are reported and patients should be monitored until symptoms resolve.15,16
Other Neurologic Diseases: TABLE 1 lists several specific neurologic conditions mentioned in state laws, including muscle spasms, Parkinson’s disease, amyotrophic lateral sclerosis (ALS), and severe chronic pain. Some of the listed diseases are similar between states, and some are uniquely mentioned; some state laws allow for additions to the legitimate medical treatment lists by commission approval or by petition. This table is not exhaustive; there are other legally treatable non-neurologic conditions that have not been included.1
Conclusion
Because of conflicting regulations and real or perceived lack of standardization, many healthcare professionals are understandably not comfortable with recommending cannabis as a viable treatment option. There is increasing evidence for the efficacy and safety of cannabis and its components, which should encourage legislators, industry, and leaders in healthcare professions to remove barriers to research, starting with removal of marijuana from the Schedule C-I category. In a time where patients, caregivers, and even practitioners are seeking alternative therapies and cost-effectiveness, cannabis and its components should be considered as options when seeking safe, effective therapies for treatment of neurologic diseases and their symptoms.
REFERENCES
1. Medical marijuana states and marijuana laws. Marijuana Doctors. www.marijuanadoctors.com/medical-marijuana/index. Accessed November 15, 2015.
2. Musto DF. The history of the Marihuana Tax Act of 1937. Arch Gen Psychiatry. 1972;26(2):101-108. Schaffer Library of Drug Policy. www.druglibrary.org/schaffer/hemp/history/mustomj1.html. Accessed December 5, 2015.
3. The Marihuana Tax Act of 1937. Shaffer Library of Drug Policy. http://druglibrary.org/schaffer/hemp/taxact/mjtaxact.htm. Accessed December 6, 2015.
4. Drug scheduling. Drug schedules. Drug Enforcement Administration (DEA). www.dea.gov/druginfo/ds.shtml. Accessed November 15, 2015.
5. American Pharmacists Association. In Connecticut, medical cannabis must be dispensed by pharmacists. Pharmacy Today. June 1, 2015. www.pharmacist.com/connecticut-medical-cannabis-must-be-dispensed-pharmacists. Accessed November 15, 2015.
6. American Pharmacists Association. Louisiana pharmacists await details about dispensing marijuana. Pharmacy Today. July 6, 2015. www.pharmacist.com/louisiana-pharmacists-await-details-about-dispensing-marijuana. Accessed November 15, 2015.
7. Sulak D. Introduction to the endocannabinoid system. NORML. http://norml.org/library/item/introduction-to-the-endocannabinoid-system. Accessed November 14, 2015.
8. Borgelt L. Pharmacology of cannabis and physiologic effects of phytocannabinoids. The Medical Cannabis Institute. Society of Cannabis Clinicians; 2014. https://themedicalcannabisinstitute.org/course/scc-pharmacology-of-cannabis-and-physiologic-effects-of-phytocannabinoids. Accessed November 14, 2015.
9. Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2014;46(1):86-95.
10. dos Santos RG, Hallak JE, Leite JP, et al. Phytocannabinoids and epilepsy. J Clin Pharm Ther. 2015;40(2):135-143.
11. Pertwee RG. Chapter 6: Known pharmacological actions of delta-9-tetrahydrocannibinol and of four other chemical constituents of cannabis that activate cannabinoid receptors. In: Pertwee RG, ed. Handbook of Cannabis. New York, NY: Oxford University Press; 2014:115-136.
12. The Endocannabinoid System. Project CBD. www.projectcbd.org/endocannabinoid-system. Accessed November 14, 2015.
13. Dronabinol monograph. Lexicomp. https://online.lexi.com. Accessed November 15, 2015.
14. Nabilone monograph. Lexicomp. https://online.lexi.com. Accessed November 15, 2015.
15. Nabiximols monograph. Lexicomp. https://online.lexi.com. Accessed November 15, 2015.
16. Sativex (THC and CBD). GW Pharmaceuticals; 2014. www.gwpharm.com/Sativex.aspx. Accessed December 7, 2015.
17. Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011;163:1344-1364.
18. deCesare K. Cannabis, the plant—a phytocanna-binoid medicine. The Medical Cannabis Institute. https://themedicalcannabisinstitute.org/course/scc-cannabis-the-plant-a-phytocannabinoid-medicine. Accessed November 15, 2015.
19. Maa E, Figi P. The case for medical marijuana in epilepsy. Epilepsia. 2014;55(6):783-786.
20. Mechoulam R, Panikashvili D, Shohami E. Cannabinoids and brain injury: therapeutic implications. Trends Mole Med. 2002;8(2):58-61.
21. Mark LP, Prost RW, Ulmer JL, et al. Pictorial view of glutamate excitotoxicity: fundamental concepts for neuroimaging. AJNR Am J Neuroradiol. 2001;22(10):1813-1824.
22. Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (-)delta-9-tetrahydrocannibinol are neuroprotective antioxidants. Proct Natl Acad Sci USA. 1998;95(14):8268-8273.
23. Van der Stelt M, Veldhuis WB, van Haaften GW, et al. Protection of rat brain by anandamide against neuronal injury. J Neurosci. 2001;21(17):8765-8771.
24. Knezevich E, Wu Y. Marijuana for the treatment of seizure disorders. US Pharm. 2015;40(1):24-28. www.uspharmacist.com/content/c/52517/. Accessed December 5, 2015.
25. Devinsky O, Cilio MR, Cross H, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55(6):791-802.
26. Cilio MR, Thiele EA, Devinsky O. The case for assessing cannabidiol in epilepsy. Epilepsia. 2014:55(6):787-790.
27. Gupta S. Why I changed my mind on weed. CNN. August 8, 2013. www.cnn.com/2013/08/08/health/gupta-changed-mind-marijuana/index.html. Accessed November 15, 2015.
28. Young S. Marijuana stops child’s severe seizures. CNN. August 7, 2013. www.cnn.com/2013/08/07/health/charlotte-child-medical-marijuana/. Accessed November 15, 2015.
29. Epidiolex. GW Pharmaceuticals. www.gwpharm.com/Epidiolex.aspx. Accessed November 15, 2015.
30. Malfitano AM, Proto MC, Bifulco M. Cannabinoids in the management of spasticity associated with multiple sclerosis. Neuropsyciatr Dis Treat. 2008;4(5):847-853.
31. Killestein J, Hoogervorst EL, Reif M, et al. Safety, tolerability, and efficacy of orally administered cannabinoids in MS. Neurology. 2002;58(9):1404-1407.
32. Robson PJ, Wade DT, Makela PM, House H. Cannabis medical extracts (CME), including cannabidiol, alleviated neurogenic symptoms in patients with multiple sclerosis and spinal cord injury. 12th Annual Symposium on the Cannabinoids. Burlington, VT: International Cannabinoid Research Society; July 11-13, 2002:56. http://cannabinoidsociety.org/HTML/ICRS.2002.symposium.pdf. Accessed December 6, 2015.
33. Vaney C, Heinzel-Gutenbrunner M, Jobin P, et al. Efficacy, safety and tolerability of an orally administered cannabis extract in the treatment of spasticity in patients with multiple sclerosis: a randomised, double-blind, placebo-controlled, crossover study. Mult Scler. 2004;10(4):417-424.
34. Collin C, Ehler E, Waberzinek G, et al. A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol Res. 2010;32(5):451-459.
To comment on this article, contact rdavidson@uspharmacist.com.





