US Pharm. 2018;43(11):30-32.
Seizures are sudden, uncontrolled electrical impulses that occur in the brain. Epilepsy, a type of seizure disorder, is a neurological condition that may result from such disturbances.1 Though the etiology is typically unknown, epilepsy is traditionally diagnosed after a person has had at least two seizures of unknown etiology.1 Seizures may occur in a pattern or because of triggers; severity depends on the trigger’s origin and the electrical activity observed. Additionally, temporary symptoms may occur, such as loss of awareness or consciousness and disturbances of movement, sensation, mood, or other cognitive functions. As a result, those affected tend to have more physical limitations, such as fractures or bruising from seizure-related injuries and higher rates of psychological conditions.
Epidemiology
Epilepsy is the fourth most common neurological disease.1 Approximately 50 million people suffer from epilepsy, and it appears to be more prevalent in low- and middle-income countries. Females have a slightly lower incidence of epilepsy when compared with males, and children have a greater propensity for seizures than adults.1
Diagnosis
Diagnosing seizures requires a medical history review, including a description of symptoms or specific characteristics of the event. Neurological exams and several other tests may be ordered for confirmation.2 Common tests include:
Electroencephalogram (EEG): EEG observes electrical activity through monitoring brain waves. This is the most common test used to diagnose epilepsy because of its ability to observe abnormal patterns, even when no seizures exist.
CT scan: CT scans use x-rays to observe images of the brain.
Magnetic resonance imaging (MRI): A MRI employs powerful magnets and radio waves to produce a detailed image of the brain.2
Traditional Treatments
Specialized care is necessary when developing an optimal treatment plan. It is important to consider the following factors when selecting the best treatment option: age, type, and frequency of seizures; the time of day of seizure activity; and previously prescribed medications.3,4
Although anticonvulsants are the mainstay of treatment, dosing and administration vary from person to person.2
Surgery
Typically reserved as a last resort, surgery may be considered once aggressive treatment with medications has failed.3 Benefits and risks must be carefully evaluated.
Device
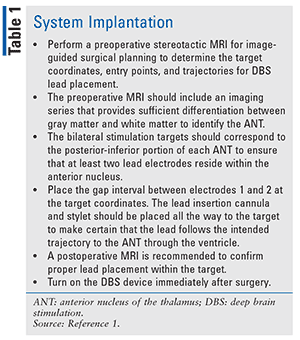
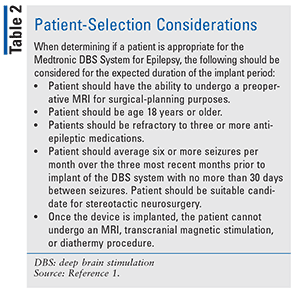
Failure to control seizures with medications and the serious risks associated with potentially curative surgeries have led to other medical advances. The Medtronic Deep Brain Stimulation (DBS) System for Epilepsy, approved by the FDA in April 2018, is a device for patients who have frequent, disabling, partial-onset seizures that have not responded positively to antiepileptic medications. The device is used in conjunction with antiepileptic drugs to decrease seizure episodes. A neurostimulator is implanted under the skin of the upper chest to send a constant stream of tiny electrical pulses to affected areas of the brain. The DBS device has not been evaluated in patients with less-frequent seizures.5 Instructions for system implantation and device selection criteria may be found in TABLES 1 and 2, respectively.5
Patient Counseling
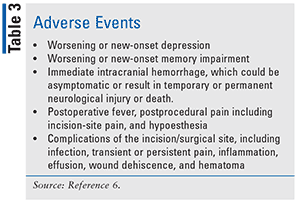
Physicians should notify patients that it takes time to achieve a maximum therapeutic effect with the Medtronic device. Before implantation, patients should be informed of possible adverse effects (TABLE 3). After initiation, a keeping a seizure diary or counting seizures on the patient programmer—a handheld device that allows the patient to record a seizure event—are essential to optimize device effectiveness.5
Efficacy
A clinical study evaluating the safety and effectiveness of bilateral stimulation of the anterior nucleus of the thalamus was conducted using the Medtronic DBS System for Epilepsy. The system was used as adjunctive therapy in adults diagnosed with epilepsy (partial-onset seizures), with or without secondary generalization, who were drug-resistant to three or more antiepileptic drugs (AEDs).5
The Stimulation of the Anterior Nucleus of the Thalamus for Epilepsy (SANTE) study was a prospective, randomized, double-blind trial involving subjects who had an average of six or more partial seizures per month. Additionally, participants had to be refractory to at least three AEDs and be taking one to four AEDs at the time of enrollment.6 Enrollment included 157 patients; however, only 110 were implanted with the device. Of the 110 patients, 109 were randomized and monitored for 13 months, up to the final completion date of the study.6 All participants continued to receive their normal AEDs throughout the trial. The blinded phase and the SANTE study lasted for 7 years and included data from 17 centers located in the United States.6
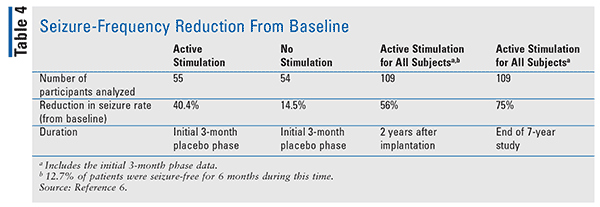
During the initial 3 months, the active group received neurostimulation and was monitored for a reduction in seizure rates when compared with the control group, who received no stimulation.7 Results demonstrated a 40.4% and 14.5% reduction in seizures in the active and control groups, respectively. Seizures were reduced by a median of 56% when compared with the baseline after 2 years of implantation. At Year 7, both quality-of- life and seizure-severity scales demonstrated statistical improvements from baseline. Additionally, measures of executive function and attention scores improved. There was no significant worsening of depression scores or cognitive declines observed in either the blinded phase or at Year 7.6,7 Moreover, by the end of the study, the frequency of seizures had been reduced by 75% (TABLE 4).6,7
Conclusion
The Medtronic DBS device is more effective than medication alone at reducing the frequency of seizures. Device effectiveness is supported by data from the SANTE study. For more information on the device, please consult the following website: www.medtronic.com/us-en/healthcare-professionals/therapies-procedures/neurological/deep-brain-stimulation.html.
REFERENCES
1. World Health Organization. Epilepsy. www.who.int/news-room/fact-sheets/detail/epilepsy. Accessed August 13, 2018.
2. Epilepsy Foundation. Diagnosis: what tests are needed? www.epilepsy.com/learn/diagnosis/diagnosis-101/what-tests-are-needed. Accessed August 14, 2018.
3. Mayo Clinic. Epilepsy: diagnosis & treatment. www.mayoclinic.org/diseases-conditions/epilepsy/diagnosis-treatment/drc-20350098. Accessed August 14, 2018.
4. Johns Hopkins Medicine. Epilepsy Center: Treatment. www.hopkinsmedicine.org/neurology_neurosurgery/centers_clinics/epilepsy/treatment/medications.html. Accessed August 14, 2018.
5. FDA. Medtronic DBS System for Epilepsy-P960009/S219. www.fda.gov/medicaldevices/productsandmedicalprocedures/deviceapprovalsandclearances/recently-approveddevices/ucm606550.htm. Accessed August 13, 2018.
6. U.S. National Library of Medicine. Clinical Trials: SANTE-Stimulation of the Anterior Nucleus of the Thalamus for Epilepsy. https://clinicaltrials.gov/ct2/show/study/NCT00101933. Accessed August 19, 2018.
7. Medscape. SANTE trial of deep brain stimulation in epilepsy. www.medscape.com/viewarticle/718845#vp_1. Accessed August 19, 2018.
To comment on this article, contact rdavidson@uspharmacist.com.