US Pharm. 2014;39(4):21-22.
Diabetes is one of the most common chronic diseases in the world, affecting some 23.6 million individuals in the United States.1,2 Although there have been several advancements in drug therapy and glucose-monitoring systems, patients and healthcare providers still face a dilemma in sustaining ideal blood glucose levels. Further, patient compliance also remains a major area of concern for healthcare providers. Nonetheless, insulin therapy remains a viable treatment modality for type 1 and type 2 diabetes mellitus. In order for insulin therapy to provide clinical benefits, such as reductions in glycosylated hemoglobin (HbA1c), self-monitoring of blood glucose and optimal dosing of insulin are essential.
Insulin Pumps
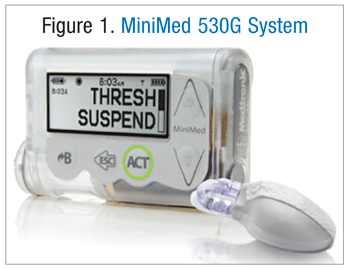
Over the course of several years, technological advances have continued to improve strategies for the administration of insulin and monitoring of blood glucose. The original insulin pump was introduced more than 30 years ago, and it allowed patients to achieve more physiological replacement of insulin through the use of mechanical delivery systems.1 Additionally, though only available for the past 10 years, continuous glucose monitors afford uninterrupted measurements of interstitial fluid glycemic levels.1 Recently, evolution of insulin pumps and continuous glucose monitors has led to the development of a platform for a combined glucose monitor and insulin delivery system: the MiniMed 530G Enlite (FIGURE 1), a sensor-augmented insulin pump that has the ability to monitor moment -to-moment glucose concentration changes—a function that has not been available when employing intermittent blood glucose self-monitoring (BGSM) systems.
Traditional Versus CGMS
Established in 1949, Medtronic is the world’s fourth largest medical device company and is well known for its advanced MiniMed continuous glucose-monitoring systems (CGMS). A few trials exist that compare standard systems and CGMS. For example, in a trial conducted by Sachedina and Pickup, the performance of the Medtronic-MiniMed CGMS was compared to that of BGSM systems to assess accuracy, reliability, and measurement of glycemic control in patients with type 1 diabetes.3 The authors recruited 18 patients with type 1 diabetes, nine of whom were males between the ages of 29 and 67 years. Glucose was monitored in study participants for up to 72 hours with CGMS, while patients with standard BGSM systems were required to test eight times daily. Study results demonstrated that 95% and 97% of paired noncalibration samples were within the clinically acceptable zones of the Clarke and error and consensus grids, respectively. The authors reported a median bias of 0.1 mmol/L and a 15% relative bias. Sensor failure rate was 28% for sensors that were initially inserted; however, CGMS detected significantly more hypoglycemia and postprandial hyperglycemia than BGSM systems. From this study, it may be concluded that CGMS have a greater clinical accuracy when compared to BGSM systems and offer patients with type 1 diabetes promising glucose control.3
MiniMed 530G Enlite System
Approved by the FDA as the first artificial pancreas device, the MiniMed 530G Enlite is a CGMS that mimics insulin delivery in a healthy pancreas. With its advanced technology, the system continuously monitors glucose levels and automatically adjusts insulin delivery with little to no patient interaction.
There are three primary parts required for the system to operate effectively: the insulin pump, the integrated continuous glucose monitor and the Enlite sensor, and the advance algorithm, which enables threshold suspend automation. Additionally, the Enlite sensor is approved to be worn for 6 days at a time and only with the MiniMed 530G. Automatic suspension is a key feature that stops insulin delivery if the glucose has reached a preset threshold (i.e., 60 to 90 mg/dL). For example, if a patient is unable to respond to an alarm, insulin delivery will be delayed for up to 2 hours. If conscious, the patient should immediately test his or her glucose and institute appropriate dosage adjustments.
Indication and Use
The MiniMed 530G System is indicated for the continuous
delivery of basal insulin and administration of insulin boluses for
individuals
>16 years of age.4 A list of device contraindications is shown in TABLE 1.4

Clinical Efficacy: CGMS Versus Insulin Therapy
Bergenstal et al conducted a study to compare the efficacy of sensor-augmented pump therapy with a regimen requiring multiple daily insulin injections in patients with type 1 diabetes.5 Three hundred twenty-nine adults and 156 children between the ages of 7 and 70 years were randomized in a 1-year multicenter, controlled trial. Participants were included if their condition was treated with multiple daily injections, if their HbA1c was between 7.4% and 9.5%, and if primary care was established with the principal investigators or referring physicians within the 6 months prior to the study. Patients were evaluated at 3, 6, 9, and 12 months after randomization. Results illustrated that in the two study groups with an HbA1c of 8.3% at baseline, the HbA1c decreased to 7.5% and 8.1% in patients who employed pump and intermittent injection therapy, respectively. The rate at which patients experienced severe hypoglycemia while being treated with pump therapy (13.31 cases per 100 person-years) did not significantly differ from the rate of those on injection therapy (13.48 per 100 person-years). The authors concluded that sensor-augmented pump therapy demonstrated greater improvement in HbA1c levels when compared with injection therapy.5
Conclusion
The MiniMed 530G System has proven to be efficacious and convenient for patients with diabetes who require insulin therapy and continuous glucose monitoring. The MiniMed 530G Enlite is a sensor-augmented pump that mimics functions of a healthy pancreas and is one of the most advanced diabetes management tools to date for patients with diabetes who require insulin pumps. For more information about the MiniMed 530G Enlite, visit www.medtronicdiabetes.com.
REFERENCES
1. Cengiz E, Sherr JL, Weinzimer SA, et al. New-generation diabetes management: glucose sensor-augmented insulin pump therapy. Expert Rev Med Devices. 2011;8(4):449-458.
2. Centers for Disease Control. Diabetes
Public Health Resource: 2011 National diabetes fact sheet.
www.cdc.gov/diabetes/pubs/estimates11.htm. Accessed January 30, 2014.
3. Sachedina N, Pickup J. Performance
assessment of the Medtronic-MiniMed Continuous Glucose Monitoring
System and its use for measurement of glycemic control in type 1
diabetics. Diabetic Medicine. 2003;20:1012-1015.
4. FDA summary of safety and
effectiveness data (SSED).
www.accessdata.fda.gov/cdrh_docs/pdf12/P120010b.pdf. Accessed January
31, 2014.
5. Bergenstal RM, Tamborlane WV, Ahmann
A, et al. Effectiveness of sensor-augmented insulin-pump therapy in
type 1 diabetic patients. N Engl J Med. 2010;363(4):311-320.
To comment on this article, contact rdavidson@uspharmacist.com.





