US Pharm. 2018;43(5)(Specialty&Oncology suppl):2-11.
ABSTRACT: Multiple myeloma (MM) is an incurable hematologic malignancy that evolves from an asymptomatic premalignancy state. Considered a heterogeneous disease, MM has a highly variable clinical course. The diagnostic criteria, staging system, and preferred treatment regimens have undergone numerous updates over the past several years. Chromosomal abnormalities and lactate dehydrogenase have been incorporated into the Revised-International Staging System to better predict survival outcomes and to identify patients earlier in the disease course in order to prevent severe end-organ disease. Management of MM continues to evolve with novel treatment drugs and combination regimens. Primary management often includes a three-drug combination of a proteasome inhibitor, an immunomodulatory agent, and dexamethasone. Monoclonal antibodies, immunotherapies, and other novel agents are showing promising results for MM.
Multiple myeloma (MM) is a hematologic malignancy of the plasma cell that is characterized by increased secretion of monoclonal immunoglobulins (M-protein) in the bone marrow. The accumulation of malignant plasma cells results in bone destruction, which leads to bone lesions. Advances in the treatment of MM have been numerous, and median survival has increased from 3 to 6 years over the past two decades.1 Combination therapy consisting of a proteasome inhibitor (PI), an immunomodulatory drug, and dexamethasone (DXM) is widely accepted as first-line therapy. Autologous stem-cell transplantation (SCT) remains fundamental to the management of MM, although allogeneic SCT is being considered for selected patients. As treatment evolves based on advances in pharmacology and supportive care, pharmacists will continue to play an integral role in the care of MM patients.
Etiology and Pathogenesis
MM is a common hematologic malignancy in the United States; it accounts for an estimated 30,280 new cases and 12,950 deaths and in 2017 constituted 1.8% of all cancers and 10% of all hematologic malignancies.2 MM incidence is slightly greater in men versus women, twice as common in African Americans as in white Americans, and lowest in Asian/Pacific Islanders.1,2 Median age at diagnosis is 69 years, and 49.6% of patients survive 5 or more years after diagnosis.2
MM arises from a terminally differentiated postgerminal center plasma cell.3 The pathogenesis of myeloma is complex, and many steps in the pathway are not fully elucidated. Most cases of MM are preceded by the premalignant asymptomatic states of monoclonal gammopathy of undetermined significance (MGUS) and smoldering MM (SMM).4 The progression of MGUS to MM is approximately 1% of cases per year, whereas SMM has a much higher rate of progression of 10% of cases annually.5 Approximately 73% of SMM patients will progress to myeloma within 15 years.6 Genetic abnormalities and molecular changes are thought to contribute to cell-cycle dysregulation and lead to active myeloma.7
Myeloma is a heterogeneous disease that is based on various genetic aberrations. Many of the chromosomal abnormalities include translocations in the immunoglobulin-heavy chain of chromosome 14, aberrations in chromosomes 1, 5, 13, and 17, and trisomies.8 MM development is affected by changes in adhesion molecule expression and subsequent interactions within the complex microenvironment of the bone marrow, which induces cytokine and growth factor secretion.6 Various cytokines, including interleukin-6, interleukin-10, and insulin-like growth factor, are produced and secreted by myeloma and other cells within the bone marrow and promote proliferation of malignant cells.1,6 Novel drug therapy targets both the myeloma cell and the microenvironment.
Signs and Symptoms
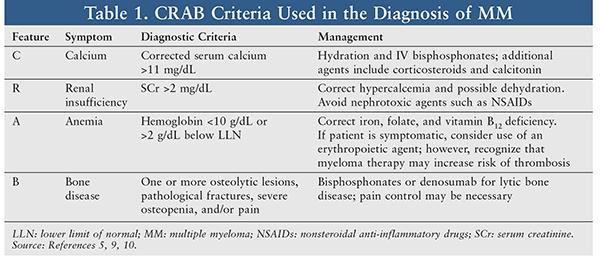
The hallmark clinical features of MM—hyperCalcemia, Renal insufficiency, Anemia, and Bone lesions—are often remembered by the mnemonic CRAB (TABLE 1).5,9 Most MM patients are symptomatic upon diagnosis; 33% of cases present with renal insufficiency, 75% have anemia commonly accompanied by fatigue, and almost 80% have bone lesions, which can be identified on x-ray or MRI.10 Patients may also experience recurrent infections or weight loss. Rarely, patients may present with hyperviscosity syndrome, which involves headache, blurred vision, epistaxis, oral bleeding, and altered mental status or confusion. Plasmapheresis may be used to alleviate symptoms of hyperviscosity.10
Diagnosis
The International Myeloma Working Group (IMWG) provides guidance on the diagnostic criteria and staging system for MM. In 2014, the IMWG updated its diagnostic criteria, noting that MM must be distinguished from other plasma-cell disorders, including immunoglobulin light chain amyloidosis and solitary plasmacytoma.9 The IMWG guidelines have been incorporated into the National Comprehensive Cancer Network (NCCN) clinical practice guidelines.10 Traditionally, MM was diagnosed on the basis of 10% or more clonal plasma cells in the bone marrow and a myeloma-defining event (i.e., evidence of end-organ damage that may be attributed to MM, which includes the CRAB features). The 2014 IMWG guidelines include three new biomarkers for patients without CRAB features, including clonal bone-marrow plasma cell percentage of 60% or greater, serum free light chain ratio 100 mg/mL or higher, and one or more focal lesions that are 5 mm or greater on MRI studies.9,10 The intent is to identify patients at high risk for disease progression earlier in the disease course in order to prevent serious end-organ damage.9 A detailed medical history and physical examination, bone-marrow biopsy, radiography, and laboratory testing are important components of diagnosis.
Staging and Prognosis
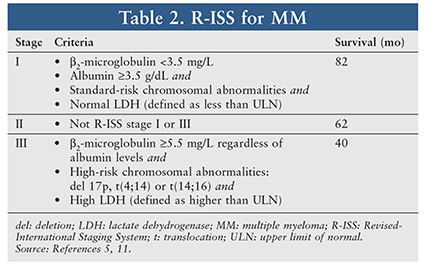
MM prognosis includes numerous tumor- and patient-specific factors. The International Staging System (ISS), published in 2005, is a simple tool that correlates MM prognosis and survival. Criteria for the three stages are based on levels of serum beta-2-microglobulin and albumin (TABLE 2).11 Over the past decade, the understanding of cytogenetics and its influence on survival have added to the prognostic factors for myeloma, which were lacking in the original ISS. In 2015, the IMWG published a revised ISS that incorporates cytogenetic factors and lactate dehydrogenase—a marker reflective of tumor burden—to the original ISS definitions of stage.11
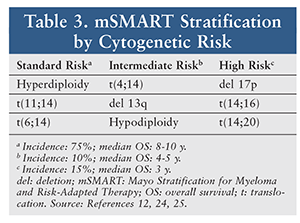
Many institutions are also incorporating a risk-adapted approach to treatment decisions. The Mayo Stratification of Myeloma and Risk-Adapted Therapy classifies risk based on cytogenetic abnormalities (TABLE 3).12 Patients with deletions of the long arm of chromosome 13 and translocations of chromosomes 4 and 14 are considered to have high-risk disease. Deletion of 17p13, which results in mutations in the tumor-suppressor protein 53, is also associated with a poorer outcome. Understanding the role of cytogenetics can assist clinicians in individualized treatment decisions based on prognosis and risk.
Response Criteria
The IMWG Uniform Response Criteria are most often used to assess response to drug therapy. Responses include stringent complete response, complete response, very good partial response, partial response, and stable disease.13 The response criteria incorporate the degree of reduction of serum and urine M-protein by electrophoresis and immunofixation, plasmacytomas, and plasma cells in bone marrow. A recent addition to the IMWG criteria involves measurements of minimal residual disease using sensitive tools such as multiparameter flow cytometry, qualitative polymerase chain reaction, and next-generation sequencing.13 This is important because the level of response has a direct correlation to survival.14
Treatment Overview
The goals of MM treatment have evolved with advances in drug therapy and more sensitive monitoring. The primary goal is to achieve a deep, long-lasting response. Additionally, therapy should control disease, minimize complications, and improve quality of life.10 Myeloma treatment depends on whether the patient is symptomatic. Patients with MGUS and SMM are usually observed, and treatment is initiated upon disease progression to active MM. There is no evidence that early treatment of asymptomatic myeloma prolongs overall survival (OS).15 Patients with symptomatic active myeloma require treatment. This treatment is patient-specific and depends on numerous factors, including cytogenetics, disease stage, age, comorbidities, and performance status. Although MM is currently incurable, advances in drug therapy may one day make cure a reality.
MM treatment involves primary therapy and assessment of SCT eligibility. Primary therapy will be followed with high-dose (HD) chemotherapy and autologous SCT and/or maintenance therapy in selected patients.10 HD chemotherapy with autologous SCT has been shown to improve overall survival (OS) in patients with MM compared with no transplantation and prolongs progression-free survival (PFS) if given immediately following primary therapy, compared with salvage therapy.10 Allogeneic SCT may be considered in selected patients, although robust data comparing outcomes with those for autologous SCT are lacking.10 The difficulty with allogeneic SCT is finding a suitable donor, and limitations include the older age and comorbidities of most MM patients. Allogeneic SCT remains of interest owing to autologous SCT’s inability to cure MM.
Regardless of transplantation status, all patients receive primary therapy with a two- or three-drug combination of an immunomodulatory agent, corticosteroid, and PI. The NCCN guidelines prefer a triple-drug regimen based on increased response rates, deeper responses, and increased OS compared with two-drug regimens.10 Two-drug regimens are reserved for frail patients and those who cannot tolerate a three-drug regimen. Primary therapy is used to reduce tumor burden and resolve the complications of MM. Following a response to primary therapy, transplant-eligible patients will undergo autologous SCT and may follow up with lenalidomide or bortezomib maintenance therapy.8 In transplant-ineligible patients, the selected regimen is typically continued until disease progression occurs; when this takes place, another regimen for relapsed disease is initiated.
Three main classes of agents are commonly used to treat MM: PIs, immunomodulatory agents, and corticosteroids. Advances in treatment regimens have minimized the role of alkylating agents. For this reason, the NCCN guidelines no longer recommend any melphalan-containing regimens.10 The newer novel drugs daratumumab and elotuzumab provide additional options for patients with relapsed/refractory disease. Because numerous combination regimens are used in the treatment of MM, commonly recommended agents including the newer novel drugs will be briefly discussed, with key points of the medications highlighted. Preferred regimens are found in the NCCN guidelines.10
PIs: These agents are the backbone of many MM regimens. Currently, three PIs are commercially available: bortezomib, carfilzomib, and ixazomib. These novel agents inhibit the 26S proteasome, leading to cell-cycle arrest and apoptosis.16 Originally approved for relapsed/refractory MM, bortezomib-based combinations are now used as primary therapy in newly diagnosed transplant-eligible and -ineligible patients.10 Bortezomib may also be used as maintenance therapy in intermediate- and high-risk MM patients. The toxicity profile includes myelosuppression, neurotoxicity (i.e., peripheral neuropathy), and increased risk of herpes simplex or zoster infections. Prophylaxis with an antiviral is recommended to reduce the risk of herpes reactivation with PI therapy.10,16 Although bortezomib was initially approved for IV use, the SC injection is now recommended based on decreased peripheral neuropathy and no differences in treatment outcomes.10
Carfilzomib and ixazomib are second-generation PIs that may be used for primary therapy in clinical trials and in relapsed/refractory disease. Ixazomib, the first oral PI, offers patients an all-oral triple-drug regimen with lenalidomide and DXM. The adverse-effect (AE) profile is similar to that for bortezomib; however, there may be a decrease in neurotoxicity with second-generation agents.10,16 Carfilzomib is administered in escalating doses and with premedications and hydration to mitigate tumor lysis syndrome and renal toxicity. Importantly, carfilzomib may be associated with serious cardiac and pulmonary toxicities, including heart failure, ischemia, and pulmonary hypertension. Careful monitoring is required, and if these toxicities are suspected, hydration should be minimized and carfilzomib should be discontinued or withheld.16
Immunomodulatory Drugs: Immunomodulatory drugs are often added to the PI base. Thalidomide, lenalidomide, and pomalidomide are three immunomodulatory drugs approved for treatment of myeloma. In practice, thalidomide has been largely replaced by lenalidomide because the newer agent has increased potency and fewer AEs. The mechanism of action is unknown, but these drugs are believed to inhibit proinflammatory cytokines and stimulate natural killer cells.16
The regimen of bortezomib, lenalidomide, and DXM is widely used for both transplant-eligible and -ineligible MM patients. Because lenalidomide may impair stem-cell mobilization, the IMWG recommends early collection of stem cells, preferably within the first four cycles of therapy.10 All three agents are available in oral formulations and are dosed once daily. Significant AEs include black box warnings for thromboembolism and the potential for severe birth defects and fetal death.16 To reduce the risk of thromboembolic events, patients should receive thromboprophylaxis. This may consist of anticoagulation or full-dose aspirin depending on the patient’s risk factors and selected drug therapy.10
Lenalidomide is also widely used as maintenance therapy following transplantation or as primary therapy in transplant-ineligible patients to prolong PFS. Lenalidomide may cause an increase in secondary malignancies, including hematologic and solid tumors, particularly in patients receiving a melphalan-containing transplant.10,16 The risks and benefits should be discussed with patients. Some clinicians recommend limiting lenalidomide maintenance therapy to 2 years; however, the optimal duration remains to be determined.17 Unlike the other antimyeloma agents and because of the contraindications, the drugs are commercially available through restricted-distribution programs, and enrollment in their respective Risk Evaluation and Mitigation Strategies (REMS) programs is required of physicians, pharmacies, and patients.16
Pomalidomide has activity in lenalidomide- and bortezomib-treated patients.17 Pomalidomide may have fewer myelosuppressive effects and cause less skin rash, constipation, and neuropathy compared with lenalidomide and thalidomide.17
Corticosteroids: A corticosteroid—specifically, DXM—is added to almost all primary and relapsed/refractory myeloma regimens. The corticosteroid dose is often quite high, compared with treatment of other diseases, and it is given on a pulse basis.10 Pharmacists should be aware of the multitude of AEs associated with corticosteroids, including weight gain, hyperglycemia, sleep disorders, and mood changes and other psychiatric symptoms.18,19 Patients should be counseled on potential AEs, as adherence to corticosteroids is essential in the treatment of myeloma. Patients should be instructed to contact their provider if symptoms are affecting their adherence; dose reductions, changes in therapy, or symptom-management interventions (i.e., glucose control) may be necessary.18,19
Additional Agents: Increased understanding of myeloma and the microenvironment has led to a plethora of novel antimyeloma drugs. Because of the lack of cure, most novel drugs are initially studied in the relapsed/refractory disease setting. Two monoclonal antibodies (Mabs) have been added to the antimyeloma armamentarium for relapsed/refractory MM. Daratumumab (Darzalex) was granted accelerated FDA approval in 2015 for patients who have received at least three prior lines of therapy, including a PI and an immunomodulatory agent.16 Daratumumab is an Mab that targets CD38 on myeloma cells. CD38 is overexpressed on myeloma cells, is considered a regulator of cell adhesion, and is involved in tumorigenesis.20 Daratumumab may be used alone or in combination with DXM and bortezomib or with lenalidomide or pomalidomide. AEs include neutropenia, thrombocytopenia, and infusion reactions; therefore, premedication with a corticosteroid, acetaminophen, and antihistamine is recommended.16 As with PIs, antiviral prophylaxis is recommended to prevent herpes reactivation. The antiviral should begin within 1 week of daratumumab initiation and should continue for 3 months following treatment.16 Daratumumab may also interfere with RBC antibody screening, so patients should be typed and screened prior to therapy initiation.16
Elotuzumab (Empliciti) is a humanized Mab that targets CS1, which is a glycoprotein primarily expressed on myeloma cells. CS1 may also be referred to as signaling lymphocytic activation molecule F7.16,20 Elotuzumab has been studied in combination with DXM and lenalidomide or bortezomib. AEs include infusion reactions (which requires premedications) and increased risk of infection.16
Panobinostat (Farydak) is an oral pan-deacetylase inhibitor used in combination with bortezomib and DXM. Panobinostat has a REMS program owing to the black box warnings on severe diarrhea (25% of patients) and the cardiac toxicities of cardiac ischemic events (4%) and arrhythmias (12%).21 Additional AEs include pancytopenia, including fatal gastrointestinal and pulmonary hemorrhages, and hepatotoxicity. Panobinostat is teratogenic, so women and men should take steps to avoid pregnancy for at least 3 months and 6 months, respectively, following the last dose of panobinostat.21
Recently, based on promising early studies, much excitement has been generated about cellular immunotherapies such as chimeric antigen receptor T-cell therapy.22
Supportive Care
Supportive care is a necessary component of MM management. Adjunctive medications can improve quality of life and minimize CRAB feature–associated complications. Because bone lesions are a hallmark characteristic of MM, denosumab or IV bisphosphonates—either zoledronic acid or pamidronate—are recommended for all patients with symptomatic MM.10 Patients should have a dental examination prior to starting bisphosphonate therapy and be monitored routinely for osteonecrosis of the jaw. In addition to skeletal-lesion prevention, the other complications of CRAB should be appropriately managed (TABLE 1). Thromboprophylaxis should be considered when patients are on an immunomodulatory drug combination, and herpes prophylaxis with an antiviral is required for patients receiving daratumumab or a PI.10 Clinicians should refer to the NCCN Clinical Practice Guidelines for Venous Thromboembolic Disease for direction on appropriate management.23
The Pharmacist’s Role
Pharmacists can play a critical role in managing MM. They can provide patient counseling, identify AEs, emphasize medication adherence, and offer supportive-care recommendations to optimize outcomes and quality of life. Many of the newer agents—including ixazomib, lenalidomide, and DXM—are oral formulations, so community pharmacists are essential in providing consultation. Risks to be discussed are specific to the individual medications but may include neurotoxicity, secondary malignancies, thromboembolisms, and birth defects. In patients receiving care in the hospital or an outpatient infusion center, appropriate laboratory work should be evaluated. Many patients have poor renal function, anemia, and hypercalcemia; therefore, supportive-care measures and dose adjustments may be necessary. Knowledge of the complex disease process of myeloma will enable pharmacists to better understand the treatment goals and provide the care their patients require.
REFERENCES
1. Röllig C, Knop S, Bornhäuser M. Multiple myeloma. Lancet. 2015;385:2197-2208.
2. National Cancer Institute. Cancer stat facts: multiple myeloma. https://seer.cancer.gov/statfacts/html/mulmy.html. Accessed November 28, 2017.
3. Anderson KC, Carrasco RD. Pathogenesis of myeloma. Annu Rev Pathol. 2011;6:249-274.
4. Landgren O, Kyle RA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412-5417.
5. Rajkumar SV. Updated diagnostic criteria and staging system for multiple myeloma. 2016 ASCO Educational Book. https://meetinglibrary.asco.org/record/50942/edbook#fulltext. Accessed November 28, 2017.
6. Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356:2582-2590.
7. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046-1060.
8. Fonseca R, Bergsagel PL, Drach J, et al. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia. 2009;23:2210-2221.
9. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15: e538-e548.
10. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Multiple myeloma. Version 3.2018. www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf. Accessed November 28, 2018.
11. Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised International Staging System for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33:2863-2869.
12. Mikhael JR, Dingli D, Roy V, et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines 2013. Mayo Clin Proc. 2013;88:360-376.
13. Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328-e346.
14. Hanbali A, Hassanein M, Rasheed W, et al. The evolution of prognostic factors in multiple myeloma. Adv Hematol. 2017;2017:4812637.
15. Kyle RA, Durie BG, Rajkumar SV, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121-1127.
16. Clinical Pharmacology [online database]. Tampa, FL: Elsevier; 2017. www.clinicalpharmacology.com. Accessed November 28, 2017.
17. Lacy MQ, Hayman SR, Gertz MA, et al. Pomalidomide (CC4047) plus low-dose dexamethasone as therapy for relapsed multiple myeloma. J Clin Oncol. 2009;27:5008-5014.
18. Lipe B, Vukas R, Mikhael J. The role of maintenance therapy in multiple myeloma. Blood Cancer J. 2016;6(10):e485.
19. Faiman B, Bilotti E, Mangan P, et al. Steroid-associated side effects in patients with multiple myeloma: consensus statement of the IMF Nurse Leadership Board. Clin J Oncol Nurs. 2008;12(suppl 3):53-63.
20. Naymagon L, Abdul-Hay M. Novel agents in the treatment of multiple myeloma: a review about the future. J Hematol Oncol. 2016;9:52.
21. Farydak (panobinostat) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corp; June 2016.
22. Ghosh A, Mailankody S, Giralt SA, et al. CAR T cell therapy for multiple myeloma: where are we now and where are we headed? Leuk Lymphoma. 2017;Nov 6:1-12 [Epub ahead of print].
23. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Cancer-associated venous thromboembolic disease. Version 1.2017. www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Accessed November 28, 2017.
24. Rajkumar SV, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc. 2016;91:101-119.
25. Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:719-734.
To comment on this article, contact rdavidson@uspharmacist.com.





