US Pharm. 2020;45(3):9-10.
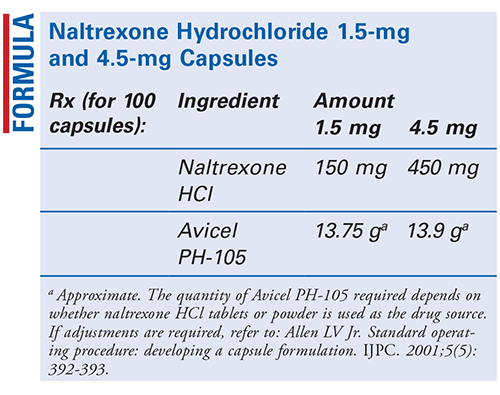
Method of Preparation: Calculate the quantity of each ingredient for the amount to be prepared. Accurately weigh each ingredient. Geometrically, incorporate the naltrexone hydrochloride (HCl) powder into the Avicel PH-105 (microcrystalline cellulose) powder until the mixture is thoroughly blended. Encapsulate the required number of capsules. Package and label.
Use: This preparation has been used in the treatment of fibromyalgia (musculoskeletal pain), psoriasis (4.5-mg capsules), complex regional pain syndrome type I, and other types of pain.1-3
Packaging: Package in tight, light-resistant containers.
Labeling: Keep out of reach of children. Keep at room temperature. Discard after ____ [time period].
Stability: A beyond-use date of at least 6 months may be used for this preparation. One study showed the 1.5-mg capsules to be stable for 1 year.1,4
Quality Control: Quality-control assessment can include weight–overall average weight, weight–individual weight variation, dissolution of capsule shell, disintegration of capsule content, active-drug assay results, physical appearance (color, uniformity, extent of fill, locked), and physical stability (discoloration, changes).5,6
Discussion: Naltrexone has been used for quite some time in patients overcoming alcohol and opioid dependency. An interesting and unexpected effect of naltrexone is its action in pain relief for different disorders. In fact, on PubMed, the search term “naltrexone AND pain” yields more than 1,350 references covering numerous types of pain. A notable aspect of naltrexone use in pain patients is that the dose is only about one-tenth of the normal dose. Since naltrexone HCl is available in 50-mg tablets, low-dose naltrexone must be compounded for patients.
Naltrexone HCl (Revia, Vivitrol, C20H23NO4.HCl, MW 377.86) is a narcotic opioid antagonist. It occurs as white crystals with a melting point of about 275°C. Naltrexone is soluble 0.1 g in approximately 1 mL water at 25°C. It must be preserved in tight containers. Naltrexone has little or no agonist activity, and its opioid antagonist activity is reported to be two to nine times that of naloxone and 17 times that of nalorphine. Naltrexone is absorbed rapidly and almost completely after oral administration, but it undergoes extensive first-pass metabolism in the liver; only 5% to 20% of an orally administered dose reaches systemic circulation unchanged.7
Microcrystalline cellulose [Avicel, MW 36,000, (C6H10O5)n where n = 220] is widely used in pharmaceuticals as an adsorbent, suspending agent, tablet and capsule diluent, and tablet disintegrant. It is a purified, partially depolymerized cellulose that occurs as a white, odorless, tasteless, crystalline powder that is composed of porous particles. Commercially, microcrystalline cellulose is available in different particle size ranges and moisture grades, with different properties and applications. Avicel PH-105 has a mean particle size of 20 µm, passing through a 400 mesh sieve, and a moisture content of less than 5%. Microcrystalline cellulose does not melt, but it will char at 260°C to 270°C. It is practically insoluble in water, dilute acids, and most organic solvents but is slightly soluble in 5% w/v sodium hydroxide solution. Microcrystalline cellulose is listed as incompatible with strong oxidizing agents. A stable but hygroscopic material, microcrystalline cellulose should be stored in well-closed containers in a cool, dry place.8
REFERENCES
1. Cote B, Ross B, Fortner J, Rao D. The use and utility of low-dose naltrexone capsules for patients with fibromyalgia. IJPC. 2018;22(3):252-256.
2. Muller G, Grieshaber R, Talley JF, et al. Compounded low-dose naltrexone for the treatment of guttate psoriasis: a case report. IJPC. 2018;22(4):270-278.
3. Sturn KM, Collins M. Low-dose naltrexone: a new therapy option for complex regional pain syndrome type I patients. IJPC. 2016;20(3):197-201.
4. U.S. Pharmacopeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; February 2020.
5. Allen LV Jr. Standard operating procedure for performing physical quality assessment of powder-filled, hard-gelatin capsules. IJPC. 1999;3:232-233.
6. Allen Jr LV. Summary of quality-control testing for sterile and nonsterile compounded preparations, part 1: physical and chemical testing. IJPC. 2019;23(3):211-216.
7. Allen LV Jr, ed. Remington: The Science and Practice of Pharmacy. 22nd ed. London, England: Pharmaceutical Press; 2012:1612.
8. Quinn ME, Sun CC. Microcrystalline cellulose. In: Sheskey PJ, Cook WG, Cable CG, eds. Handbook of Pharmaceutical Excipients. 8th ed. London, England: Pharmaceutical Press; 2017:194-198.
To comment on this article, contact rdavidson@uspharmacist.com.





