US Pharm. 2017;42(4):HS16-HS20.
ABSTRACT: Necrotizing soft-tissue infections (NSTIs) are a type of serious bacterial infection that requires rapid recognition and intervention by physicians and surgeons in combination with timely antibiotic treatment. These infections may have a polymicrobial cause, but methicillin-resistant Staphylococcus aureus is involved in most cases. In treating NSTIs, time is of the essence in order to avoid complications such as amputation, and new drugs have recently been added to the list of treatment options. These novel antibiotics include tedizolid (Sivextro), oritavancin (Orbactiv), and dalbavancin (Dalvance). Notably, the long half-life of both dalbavancin and oritavancin allows for either a single-dose regimen or once-weekly dosing.
Necrotizing soft-tissue infection (NSTI) is a broader term than necrotizing fasciitis because, in NSTI, the infection may extend beyond the fascia. Colloquial terms include “flesh-eating bacteria” and “flesh-eating infection.” This type of infection was first noted in the 5th century bc by Hippocrates, who described it as follows: “Many were attacked by the erysipelas all over the body when the exciting cause was a trivial accident...flesh, sinews, and bones fell away in large quantities...there were many deaths.”1 Since that time, other descriptive terms have been used to characterize NSTI, such as “phagedaenic ulcer, phagedena gangrenous, gangrenous ulcer, malignant ulcer, putrid ulcer, or hospital gangrene.”2 The term gangrene is still used, as in Fournier gangrene, which is necrosis of the perineum and scrotum.3
Epidemiology
The risk of NSTI remains low overall, with approximately 1,000 cases per year occurring in the United States. This number may be rising, however, perhaps because of greater awareness and recognition of the disease state, increasing antibiotic resistance, or enhanced bacterial virulence capability.4
Etiology
NSTIs are classified by anatomical location (e.g., Fournier gangrene), but they most commonly occur in the extremities, perineum, and genitalia. Common bacterial causative organisms include methicillin-resistant Staphylococcus aureus (MRSA), gram-negative organisms and anaerobes, and Group A streptococci (GAS).4
Clinical Presentation and Diagnosis
The initial presentation of patients with NSTI may mimic symptoms associated with cellulitis, including erythema, swelling, fever, induration, and edema outside the area of skin changes.4 Patients may describe soreness or pain similar to that of a pulled muscle.5 Symptoms that should heighten the clinician’s suspicion of NSTI include pain that is disproportionate to findings upon examination (early in disease course), the presence of bullae, skin ecchymosis preceding skin necrosis, gas within the tissues, and cutaneous anesthesia. In addition, compared with patients with a diagnosis of cellulitis, the chronology of infection in patients with NSTI points to a much more rapid disease progression.4 Therefore, patients suspected of having cellulitis should have the diagnosis of necrotizing fasciitis ruled out.6 For an infection to occur, the bacteria must enter the bloodstream, usually through an inciting wound (a break in the epithelial or mucosal surface) such as a minor cut or scratch, insect bite, skin ulcer, or postsurgical wound; however, occasionally such injuries cannot be identified. Risk factors for NSTI include an immunocompromised state (AIDS, steroid therapy), obesity, alcohol abuse, cancer, diabetes mellitus, peripheral vascular disease, kidney disease, malnutrition, and other chronic conditions. Active parenteral drug use (IV or subcutaneous injection of illicit substances) has also been identified as a risk factor, particularly in the urban setting.7
Guidelines Review
The 2014 Infectious Diseases Society of America (IDSA) Practice Guidelines for the Diagnosis and Management of Skin and Soft Tissue Infections contain information about managing all types of skin and soft-tissue infections, ranging from simple cellulitis to cutaneous anthrax in healthy individuals and compromised patients. Because these infections occur across all age groups, the guidelines provide recommendations for both pediatric and adult patients. A subsection of the guidelines gives information related to NSTI. Specific recommendations for treatment are provided based on a rating that indicates the strength of the evidence for the recommendation according to the IDSA/U.S. Public Health Service grading system for rating recommendations in clinical guidelines.6 The guideline provides answers to 24 clinical questions, including two questions that are related to NSTI.
The first NSTI-related question addressed by the guidelines is: What is the preferred evaluation and treatment of necrotizing fasciitis, including Fournier gangrene? The first recommendation is for prompt surgical consultation in patients with aggressive infections with signs of systemic toxicity or if there is concern for necrotizing fasciitis or gas gangrene.6 This is followed by two recommendations related to antibiotic therapy. Initial antimicrobial therapy should be broad in order to provide coverage for both single-organism and multiorganism infections. Additionally, penicillin in combination with clindamycin is recommended for documented GAS necrotizing fasciitis. All three recommendations have a rating of strong with low-quality evidence, indicating that the desirable effects clearly outweigh any undesirable effects. After the recommendations, an evidence review is given, followed by a review of the most common clinical features, microbiological causes (monomicrobial versus polymicrobial), diagnosis, and treatment.
A separate section of the guidelines focuses on the management of clostridial NSTI according to the following question: What is the appropriate approach to the evaluation and treatment of clostridial gas gangrene or myonecrosis? First, urgent surgical exploration of the suspected gas gangrene site and debridement of the involved tissue should be performed. Broad-spectrum antibiotic therapy is suggested unless there is a definitive microbiological cause. In the case of clostridial myonecrosis, penicillin in combination with clindamycin is suggested. Finally, hyperbaric oxygen therapy is not recommended. All of these suggestions have a strong recommendation, and all but the surgical recommendation have a low quality of evidence (the surgical recommendation has a moderate quality of evidence). A review of the evidence surrounding these recommendations concludes the section on NSTI.6
In addition to differing from less severe skin infections by clinical presentation and other systemic manifestations, NSTIs require a different approach to treatment. There are many varieties of NSTI, but the antibiotic treatment and surgical approach are similar for all types, and surgical intervention is the primary approach when an NSTI is expected. Most patients will require multiple visits to the operating room (OR) until debridement is no longer needed.6
Drug Treatment
Initial antibiotic selection should encompass agents with activity against a broad range of organisms, including aerobes, MRSA, and anaerobes (TABLE 1).6 Patient-specific factors such as previous antibiotic exposure, concomitant disease states, organ dysfunction, and allergies should be considered in the selection of antibiotics. As is the case in the management of most infections, good antimicrobial stewardship includes optimizing antibiotic therapy to the narrowest spectrum once a microbiological cause has been identified.
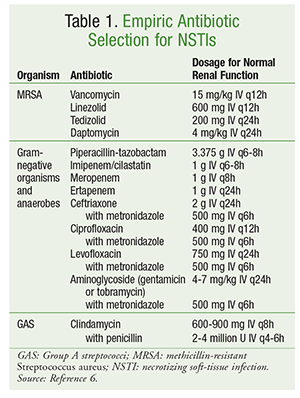
Definitive management of NSTI involves a variety of antibiotic agents. For polymicrobial infections, broad-spectrum antibiotic therapy is often continued. In the presence of infections caused by GAS, both clindamycin and penicillin should be used. The addition of clindamycin suppresses toxin and cytokine production, and the use of penicillin provides coverage in the event of clindamycin resistance to GAS. For infections caused by methicillin-susceptible S aureus, the use of nafcillin, oxacillin, or cefazolin is preferred. Clostridial infections should be managed with clindamycin in combination with penicillin. Antibiotic therapy is typically continued until no further debridement is necessary, the patient has shown clinical improvement, and fever has been absent for 48 to 72 hours.6
Since the publication of the 2014 IDSA guidelines, several new antibiotics have been approved for use in the management of complicated skin and soft-tissue infections. These agents are tedizolid (Sivextro), oritavancin (Orbactiv), and dalbavancin (Dalvance).
Tedizolid is an oxazolidinone antibiotic similar to linezolid. It has reliable activity against MRSA and other multidrug-resistant gram-positive organisms. In contrast to the twice-daily dosing of linezolid, tedizolid is given in a single daily dose, either orally or IV. Tedizolid may have more favorable adverse-effect and drug-interaction profiles compared with linezolid.8 Although tedizolid has been studied in the management of acute bacterial skin and skin-structure infections, it has not been studied in NSTI.
Dalbavancin and oritavancin are lipoglycopeptides approved for the management of acute bacterial skin and skin-structure infections. These agents are unique in that they have a long half-life, allowing for either a single-dose regimen or a once-weekly regimen. The dosage of dalbavancin must be reduced in patients with a creatinine clearance <30 mL/minute. Oritavancin does not require any organ dysfunction–related dose adjustments, but it should not be used in patients receiving heparin because it artificially prolongs coagulation tests. Oritavancin may also increase the effects of vitamin K antagonists such as warfarin.9 Both agents have proven efficacy in the management of skin and skin-structure infections, but they have not been evaluated for use in necrotizing infections.
Adjunctive therapies for NSTI include IV immunoglobulin (IVIG) and hyperbaric oxygen therapy. IVIG is thought to be beneficial because of possible neutralization of the toxins produced in streptococcal infections. Reductions of these toxins could potentially decrease some of the organ failure, shock, and tissue destruction often seen in NSTI.6 At the time the IDSA guidelines were published (April 2014), an observational study and a trial from Europe reported some successes with the use of IVIG in streptococcal infections.6 However, based on the lack of evidence supporting efficacy, the use of IVIG is not currently recommended.
A more recent study described the effectiveness of clindamycin and IVIG against invasive GAS infections.10 Eighty-four patients met the criteria for invasive GAS disease, and IVIG was used in 14 patients, including eight with necrotizing fasciitis. Mortality was lower in patients treated with clindamycin and IVIG, but this difference was not significant. Although there was not a statistical difference in mortality when IVIG was added to clindamycin, the authors suggested that there may be a possible benefit in adults with more severe disease.10
Hyperbaric oxygen therapy (HBOT) has been advocated for the management of gas gangrene, but its role is controversial.6 Laboratory studies have shown that oxygen-rich tissue states help suppress the growth of Clostridium perfringens and increase host defenses against foreign cells, but criteria are lacking to identify patients who may benefit and the appropriate time to begin therapy.6,11 Nothing should delay emergent surgical debridement and antibiotic administration, as both are important in ensuring survival.6
Because the majority of published studies were small and underpowered, a study was conducted to evaluate data on the use of HBOT from several centers with HBOT facilities.11 Over a 3-year period, 14 HBOT facilities saw 1,583 cases of NSTI and provided adjuvant HBOT in 117 cases. The HBOT group had increased costs and a longer length of hospital stay than the control group, but it had a significantly lower hospital mortality rate (5% vs. 12%, respectively; P <.05). When severity of illness was considered, the most severely ill HBOT patients had significantly lower rates of mortality and complications, but there were no differences in length of stay or cost. A multivariate analysis demonstrated that patients who did not receive HBOT were more likely to die during hospitalization (odds ratio, 10.6; 95% CI 5.2-25.1). Each increase in severity of illness carried an increased risk of death.
This study of 14 HBOT facilities appears to show improved outcomes, especially for the sickest patients admitted directly to a center with the ability to provide this treatment.11 However, this does not allay the controversy surrounding the use of HBOT, as this investigation had several limitations. The study was conducted retrospectively using an administrative database at academic medical centers. Also, some centers may have a third party that owns the HBOT facility, making the results less generalizable to patients at those institutions. A randomized, controlled trial would be helpful in determining the ultimate benefit of this therapy.
Complications
The mortality rate associated with NSTI is as high as 30% to 70% in patients with GAS necrotizing fasciitis combined with hypotension and organ failure.6 Because treatment involves multiple visits to the OR, large wounds and possible amputations are routinely reported. Other complications, such as reconstructive surgery, sepsis, nosocomial infections, and prolonged critical care time due to the development of multisystem organ failure, are frequent complications of NSTI.12
Information about long-term effects of NSTI was gathered from in-depth interviews of 18 survivors.12,13 The investigators found that reported quality of life was significantly affected by physical function and continued pain. The effect of the experience on family and other relationships was discovered to be significant as well. Several survivors described long-term psychological consequences of the disease, including depression and posttraumatic stress.
Conclusion
Hospital pharmacists are very well suited to assist in the care of patients with NSTIs, including streamlining of care; promotion of antimicrobial stewardship; and improvements in clinical outcomes, cost reduction, complication minimization, and readmissions. Other roles of the pharmacist include identifying ideal patients for the new but more expensive therapeutic agents (e.g., oritavancin) and recognizing drug interactions (i.e., anticoagulants) with these antibiotic classes.
REFERENCES
1. Descamps V, Aitken J, Lee MG. Hippocrates on necrotising fasciitis. Lancet. 1994;344:556.
2. Loudon I. Necrotising fasciitis, hospital gangrene, and phagedena. Lancet. 1994;344:1416-1419.
3. Sarani B, Strong M, Pascual J, Schwab CW. Necrotizing fasciitis: current concepts and review of the literature. J Am Coll Surg. 2009;208:279-288.
4. Hakkarainen TW, Kopari NM, Pham TN, Evans HL. Necrotizing soft tissue infections: review and current concepts in treatment, systems of care, and outcomes. Curr Probl Surg. 2014;51:344-362.
5. CDC. Necrotizing fasciitis: a rare disease, especially for the healthy. www.cdc.gov/features/necrotizingfasciitis/. Accessed November 29, 2016.
6. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159.
7. Bosshardt TL, Henderson VJ, Organ CH Jr. Necrotizing soft-tissue infections. Arch Surg. 1996;131:846-852.
8. Kisgen JJ, Mansour H, Unger NR, Childs LM. Tedizolid: a new oxazolidinone antimicrobial. Am J Health Syst Pharm. 2014;71:621-633.
9. Roberts KD, Sulaiman RM, Rybak MJ. Dalbavancin and oritavancin: an innovative approach to the treatment of gram-positive infections. Pharmacotherapy. 2015;35:935-948.
10. Carapetis JR, Jacoby P, Carville K, et al. Effectiveness of clindamycin and intravenous immunoglobulin, and risk of disease in contacts, in invasive group A streptococcal infections. Clin Infect Dis. 2014;59:358-365.
11. Shaw JJ, Psoinos C, Ernhoff TA, et al. Not just full of hot air: hyperbaric oxygen therapy increases survival in cases of necrotizing soft tissue infections. Surg Infect (Larchmt). 2014;15:328-335.
12. Hakkarainen TW, Burkette Ikebata N, Bulger E, Evans HL. Moving beyond survival as a measure of success: understanding the patient experience of necrotizing soft-tissue infections. J Surg Res. 2014;192:143-149.
13. Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. New Engl J Med. 2005;352:1445-1453.
To comment on this article, contact rdavidson@uspharmacist.com.






