US Pharm.
2007;32(10):HS-14-HS-23.
Approximately 39.5 million people worldwide
are infected with human immunodeficiency virus (HIV).1 The standard
of care for treatment of HIV infection centers around combination
antiretroviral therapy (ART), involving the use of medications from at least
two distinct therapeutic classes, to inhibit viral replication. This approach
has been proven to slow the progression to acquired immunodeficiency syndrome
(AIDS). There are four classes of antiretrovirals currently available,
including the nucleoside reverse transcriptase inhibitors (NRTIs),
nonnucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors
(PIs), and fusion inhibitors. Combination regimens include three or more
antiretroviral agents. Therapy for HIV infection involves numerous adverse
effects and the complication of resistance development. Once patients develop
resistance to an agent in a particular class, they are likely to have
cross-resistance to other agents in the class, which minimizes therapeutic
options. This emphasizes the need for new approaches for the treatment of HIV,
including agents with novel mechanisms of action.
This article will review several emerging
antiretroviral therapies, focusing primarily on those in more advanced stages
of clinical development. These include entry inhibitors, integrase inhibitors,
and maturation inhibitors. The role of many of these agents will be as salvage
treatment for patients with numerous HIV-resistant mutations to currently
available agents, while others will be targeted as initial therapies for
treatment-naïve patients.
Entry Inhibitors: CCR5 Antagonists
An exciting approach to treatment
has involved the development of agents that inhibit the entry of HIV by
preventing the binding of viral envelope proteins to receptors on host cells.
These drugs differ from NRTIs, NNRTIs, and PIs in that they target a host cell
receptor rather than affect enzymes utilized in viral replication.
In order to gain entry into its host CD4
cell, the envelope proteins of HIV bind to a main CD4 receptor as well as a
key coreceptor (CCR5 or CXCR4)2 (FIGURE 1). The viral
membrane fuses to the CD4 cell membrane with subsequent viral entry, resulting
in infection of the host cell. Viruses that primarily use CCR5 as a coreceptor
are known as R5 tropic and are present during both the acute
seroconversion and asymptomatic phases of HIV infection.3,4 Viruses
that use only CXCR4 as a coreceptor are known as X4 tropic. These may
be more prevalent during later infection and result in more rapid progression
of HIV infection, although CD4 counts are comparable to those of patients
infected with R5 strains.3-6 Some variants of HIV utilize both the
CCR5 and the CXCR4 coreceptors; these are known as dual tropic R5/X4 or mixed
R5 and X4 viruses.3
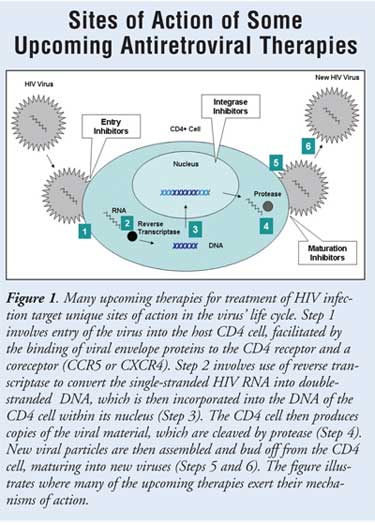
There has been great interest in the
development of treatments that antagonize these coreceptors, thereby
preventing entry and subsequent infection of CD4 cells. Strategies that target
the CCR5 receptor are in the most advanced stages of development. These
include small- molecule CCR5 inhibitors as well as monoclonal antibodies to
the CCR5 receptor.5,7 Table 1 provides a summary of those
agents and their current clinical status.
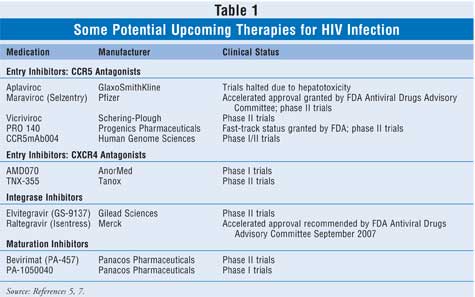
Small-molecule CCR5 antagonists include
aplaviroc, maraviroc, and vicriviroc. Trials of aplaviroc were halted in 2005
due to severe hepatotoxicity in several patients.5 As a result,
safety assessments of the other two CCR5 antagonists have closely reviewed
hepatic adverse effects, although neither has been consistently linked with
hepatotoxicity.5 Although one clinical trial involving vicriviroc
was halted in treatment-naïve patients due to virologic failure, data from a
completed phase II trial in treatment-experienced patients indicated a
significant decrease in viral load with vicriviroc as compared with placebo.
8,9 In this latter trial, there were four cases of lymphoma, the
causality of which the authors indicated was uncertain.9 The agent
with a large amount of promising data is maraviroc; these results are reviewed
below.
Data from two major clinical trials
assessing maraviroc in treatment-experienced patients was presented at the
14th Conference on Retroviruses and Opportunistic Infections in early 2007.
10,11 Together, these double-blind, placebo-controlled trials, known as
MOTIVATE 110 and MOTIVATE 211, randomized a total of 1,076 patients worldwide
to receive maraviroc twice daily, maraviroc once daily, or placebo in
combination with an optimized background regimen of currently available ART.
In both studies, maraviroc treatment resulted in significantly better viral
suppression at week 24 as compared with placebo. Patients in both maraviroc
groups also achieved significantly higher CD4 counts as compared to placebo
(MOTIVATE 1 trial: CD4 increased by 107 to 111 cells/mL from baseline in
maraviroc groups versus 52 cells/mL in placebo group, P<0.0001;
MOTIVATE 2 trial: CD4 increased by 102 to 112 cells/mL in maraviroc groups
versus 64 cells/mL in placebo group, P <0.001). Maraviroc was similar
to placebo in terms of serious adverse effects, and none of the deaths in the
studies were attributed to the study medication.
Following receipt of a fast-track
designation from the FDA in July 2005, an expanded access program was
developed by maraviroc's manufacturer the following year.12
In addition, the FDA Antiviral Drugs Advisory Committee unanimously
recommended accelerated approval for maraviroc tablets for treatment of HIV in
treatment-experienced patients in June 2007.13 The committee
mandated a minimum of five years of follow-up of viral loads, CD4 counts,
viral tropism, AIDS-defining illnesses, and mortality for patients who
demonstrated virologic failure in the phase II/III studies presented in the
new drug application.12 They also recommended that the package
insert illustrate safety concerns with the CCR5 antagonist class, including
hepatotoxicity. Ongoing studies are assessing the use of maraviroc in
treatment-naïve patients.12 The drug received full FDA
approval on August 6, 2007.14 Specific dosing information, drug
interactions, adverse effects, and special considerations for maraviroc,
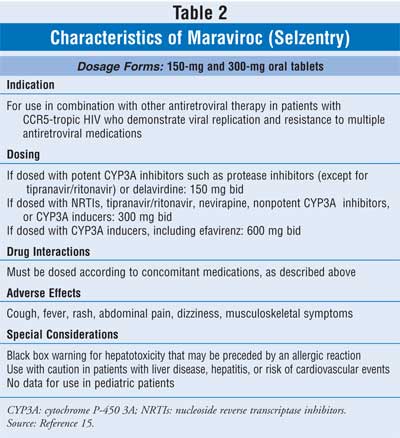
located in the package insert, are listed in TABLE 2.15
Additional CCR5 antagonists in development
include monoclonal antibodies that bind to the CCR5 receptor (TABLE 1).
PRO 140 is an injectable agent that has been granted fast-track status by the
FDA, with additional clinical trials planned in late 2007. 12,16
This agent is synergistic with small-molecule CCR5 antagonists; in addition,
it demonstrates in vitro activity against HIV resistant to the
small-molecule agents.6,16 CCR5mAb004 is another intravenous
monoclonal antibody in early development.
Many potential issues are of concern with
the CCR5 antagonist class. One is the possibility that these agents may select
for X4 tropism, which has been correlated with disease progression.3
Another is the chance that altered CCR5 interactions permitting viral escape
could occur through mutation of the virus, allowing use of an alternate
coreceptor to gain entry into the CD4 cell.3,6 In addition,
patients must be tested for coreceptor tropism prior to the use of these
agents and periodically during treatment.3 In addition, concerns
about hepatotoxicity, lymphomas, and increased susceptibility to other
infections have plagued these agents.6 Ongoing and future clinical
trials will assess these concerns to determine if they are clinically relevant.
Entry Inhibitors: CXCR4 Antagonists
These agents are in earlier
stages of clinical development relative to CCR5 antagonists (TABLE 1).
AMD070 is an orally bioavailable CXCR4 antagonist that is additive or
synergistic with some currently available ART, including enfuvirtide.3
TNX-355 has been shown to significantly decrease viral load and increase CD4
counts in treatment-experienced patients.17
Integrase Inhibitors
Integrase is a key enzyme
involved in two distinct steps of the viral replication pathway: the
processing of viral DNA strand ends and the catalyzing of the subsequent
insertion of viral DNA directly into host cell chromosomes (FIGURE 1).
These reactions are essential for viral replication. Inhibition of integrase
activity can result in the inability of HIV to infect host cells. Because
integrase inhibitors have a different mechanism of action than currently
available agents, they represent a potential additional treatment option.
Currently, two integrase inhibitors are under clinical development:
raltegravir and elvitegravir.
Data from two raltegravir phase III trials
were available in 2007.18,19 The BENCHMRK-1 and BENCHMRK-2 clinical
studies were designed to evaluate the efficacy and safety of raltegravir in
patients with demonstrated resistance to other classes of antiretroviral drug
therapy.18,19 BENCHMRK-1 enrolled participants from Europe and
Asia, and the patient population of BENCHMRK-2 was from North America and
South America. Participants in both studies had a mean CD4 cell count of 152
cells/mL. Both studies are ongoing randomized, placebo-controlled evaluations
of raltegravir 400 mg twice daily or placebo with optimized background ART.
After 16 weeks, antiretroviral efficacy was demonstrated in both studies. In
BENCHMRK-1, 61% of raltegravir-treated patients achieved a viral load of less
than 50 copies/mL compared with 33% of those receiving placebo (P
<0.001).18 CD4 count improved by 51 cells/mL in the
raltegravir-treated patients compared to placebo (P <0.001).18
Findings of BENCHMRK-2 were similar, with a viral load of less than 50
copies/mL observed in 62% and 36% of raltegravir and placebo patients,
respectively. 19 Compared to placebo, raltegravir-treated patients
had a 46 cell/mL improvement in CD4 count (P <0.001).
In both trials, adverse events were noted to be similar between groups.
18,19
The Antiviral Drug Advisory Committee of the
FDA recommended approval of raltegravir for use in combination with other
agents for treatment-experienced patients with HIV infection.20
Raltegravir was granted priority status by the FDA. In the briefing document
supplied by Merck to the FDA, a dosing regimen of 400 mg twice daily, in
combination with other unspecified agents, was recommended.21
Although raltegravir has a low potential for drug–drug interactions, a dosage
increase may be recommended when it is coadministered with rifampin,
phenytoin, or phenobarbital.21 Merck currently states that gender,
age, organ function, race, or body weight does not impact raltegravir
pharmacokinetics and that these factors will not alter drug dosing.21
The addition of raltegravir to other agents appears to be well tolerated.
21 At the time of this writing, the raltegravir specifics are only
preliminary information, and readers are advised to consult the completed
package insert when it becomes available.
Elvitegravir is currently in the phase II
stage of clinical development.7 Pharmacokinetics and dose–response
relationships have been studied in both treatment-naïve and experienced
patients.22 Pharmacodynamic studies of elvitegravir have suggested
a similar profile to PIs in that using "booster" doses of ritonavir can help
maintain therapeutic plasma concentrations of elvitegravir.
Coadministration with ritonavir 100 mg allows for once-daily elvitegravir
dosing. In an ongoing dose-ranging study, the efficacy of elvitegravir 50 mg
or 125 mg and ritonavir 100 mg daily added to standard therapy was compared to
a boosted PI regimen.23 At 24 weeks, improvements were demonstrated
in both viral load decline and CD4 count increases in patients receiving
elvitegravir with ritonavir. It was noted that elvitegravir was well
tolerated, and there was not a dose–response relationship with respect to
adverse events.23 Elvitegravir should be administered with food due
to the resulting improved bioavailability.22
Some ongoing concerns with the integrase
inhibitor drug class include the possibility of unknown safety issues and the
potential for development of resistance.24 Both agents appear to
have very few adverse effects. Additional, smaller studies have listed
headache, upset stomach, and increased liver function tests as adverse events.
While the integrase enzyme is important for the viral life cycle, it does not
appear to have an analogous role in host cell development. However, at
concentrations 10- to 20-fold higher than therapeutic levels, integrase
inhibitors may alter normal antibody production.24,25 The clinical
consequences of higher drug concentrations have not been determined.
To be most effective in preventing
resistance, integrase inhibitors should be taken in combination with other
antiretroviral agents. Analysis of viral strains from participants who have
failed raltegravir regimens has revealed several key mutation pathways. It is
not clear if resistance to raltegravir leads to cross-resistance with
elvitegravir. As with other antiretroviral drugs, selection of appropriate
combination regimens with integrase inhibitors will be essential for
preventing resistance. The importance of patient compliance with these
regimens cannot be overemphasized.
Maturation Inhibitors
Another step in the HIV viral
life cycle involves the processes related to maturation of the virus. Several
protein-processing events occur late in viral production, which are essential
for the virus to maintain its ability to replicate (FIGURE 1).
Experiments with maturation inhibitors have shown that they can inhibit viral
infectivity and replication. This specific drug class is the intellectual
property and a focus area of Panacos Pharmaceuticals; all of the following
listed agents are undergoing clinical development through Panacos.26
Bevirimat (PA-457) is undergoing phase II clinical trials. In 2006, an
Investigational New Drug Application was filed for a second-generation HIV
maturation inhibitor (PA-1050040), with phase I trials anticipated to begin in
2007. Panacos is also evaluating a potential third-generation maturation
inhibitor.
A phase IIa dose-ranging clinical study
evaluated bevirimat in HIV-positive patients who had not previously received
treatment.27 On day 11 of bevirimat therapy, there was a
statistically significant viral load reduction in those patients receiving
berivimat. A subsequent study demonstrated antiretroviral efficacy with
bevirimat, but there was a lower-than-expected bioavailability of drug in some
patients. Panacos is hoping to continue dose-escalation studies and improve
the bevirimat formulation. The manufacturer is also investigating future
agents that can potentially be effective in bevirimat-resistant HIV strains.
Conclusion
Although the increased
availability of ART options has decreased morbidity and mortality among
HIV-positive individuals in the past 10 years, management of HIV infection
continues to be a challenge. Successful care entails the lifelong use of
combination ART with optimal patient compliance to help prevent development of
resistance and AIDS-related complications. Several emerging therapies are in
the pipeline that target unique components of the viral life cycle and offer
different mechanisms of action compared with existing alternatives. Trials
have demonstrated improvements in viral load reduction and CD4 cell counts
with the addition of either entry inhibitors, integrase inhibitors, or
maturation inhibitors to standard ART. Future research will help further
define the safety profile, resistance patterns, and ultimate place in therapy
of these new drug therapies in the treatment of HIV infection.
The authors would like to acknowledge
Karen L. Houle, MS, for her assistance in the development of Figure 1.
References
1. UNAIDS. Global summary of the AIDS epidemic, December 2006. Available at: http://data.unaids.org/pub/EpiReport/2006/02-Global_Summary_2006_EpiUpdate_eng.pdf. Accessed July 3, 2007.
2. Suresh P, Wanchu A. Chemokines and chemokine receptors in HIV infection: role in pathogenesis and therapeutics. J Postgrad Med.2006;52:210-217.
3. Reeves JD, Piefer AJ. Emerging targets for antiretroviral therapy. Drugs. 2005;65:1747-1766.
4. Rusconi S, Scozzafava A, Mastrolorenzo A, Supuran CT. New advances in HIV entry inhibitors development. Curr Drug Targets-Infect Dis. 2004;4:339-355.
5. Deeks SG. Challenges of developing R5 inhibitors in antiretroviral naïve HIV-infected patients. Lancet. 2006;367:711-713.
6. Biswas P, Tambussi G, Lazzarin A. Access denied? The status of co-receptor inhibition to counter HIV entry. Expert Opin Pharmacother. 2007;8:923-933.
7. Experimental treatments for HIV/AIDS. Available at: www.hivandhepatitis.com/recent/
experimental_drugs/1.html. Accessed July 12, 2007.
8. Lederman MM, Penn-Nicholson A, Cho M, et al. Biology of CCR5 and its role in HIV infection and treatment. JAMA. 2006;296:815-826.
9. Gulick R, Su Z, Flexner C, et al. ACTG 5211: Phase II study of the safety and efficacy of vicriviroc in HIV-infected treatment-experienced subjects. Program and abstracts of the 16th International AIDS Conference; August 13-18, 2006; Toronto, Canada. Abstract ThLB0217.
10. Lalezari J, Goodrich J, DeJesus E, et al. Efficacy and safety of maraviroc plus optimized background therapy in viremic ART-experienced patients infected with CCR5-tropic HIV-1: 24-week results of a phase 2b/3 study in the US and Canada. Program and abstracts of the 14th Conference on Retroviruses and Opportunistic Infections; February 25–28, 2007; Los Angeles, CA. Abstract 104bLB.
11. Nelson M, Fätkenheuer G, Konourina I, et al. Efficacy and safety of maraviroc plus optimized background therapy in viremic, ART-experienced patients infected with CCR5-tropic HIV-1 in Europe, Australia, and North America: 24-week results. Program and abstracts of the 14th Conference on Retroviruses and Opportunistic Infections; February 25–28, 2007; Los Angeles, CA. Abstract 104aLB.
12. DIA Dispatch. Today's headline: unanimous accelerated approval recommendation for Pfizer's HIV drug maraviroc. Available at: www.diahome.org/DIAHOME/Resources/
FindPublications.aspx. Accessed June 15, 2007.
13. Press Release. Pfizer receives approvable letter from FDA for maraviroc. Available at: www.pfizer.com/pfizer/are/news_releases/index.jsp. Accessed July 9, 2007.
14. Press Release. Pfizer's Selzentry (maraviroc) tablets, novel treatment for HIV, approved by FDA. Available at: www.pfizer.com/news/pfizer_press_releases.jsp. Accessed September
10, 2007.
15. Full prescribing information. Selzentry (maraviroc) tablets. New York, NY: Pfizer Labs; August 2007.
16. Progenics announces results from first clinical trial of CCR5 inhibitor PRO 140. Available at: www.hivandhepatitis.com/recent/2007/050407_b.html. Accessed May 21, 2007.
17. Norris D, Morales J, Godofsky E, et al. TNX-355, in combination with optimized background regimen (OBR), achieves statistically significant viral load reduction and CD4 cell count increase when compared with OBR alone in phase 2 study at 48 weeks. Program and abstracts of the 16th International AIDS Conference; August 13-18, 2006; Toronto, Canada. Abstract ThLB0218.
18. Cooper D, Gatell J, Rockstroh J, et al. Results of BENCHMRK-1, a phase III
study evaluating the efficacy and safety of MK-0518, a novel HIV-1 integrase inhibitor, in patients with triple-class resistant virus. Program and abstracts of the 14th Conference on
Retroviruses and Opportunistic Infections; February 25–28, 2007; Los Angeles, CA.
Abstract 105aLB.
19, Steigbigel R, Kumar P, Eron J, et al. Results of BENCHMRK-2, a phase III study evaluating the efficacy and safety of MK-0518, a novel HIV-1 integrase inhibitor, in patients with triple-class resistant virus. Program and abstracts of the 14th Conference on Retroviruses and Opportunistic Infections; February 25–28, 2007; Los Angeles, CA. Abstract 105bLB.
20. Press Release. FDA Advisory Committee Unanimously Recommends Accelerated Approval of ISENTRESS™ (raltegravir), Merck's Investigational Oral Integrase Inhibitor, for Treatment of HIV. Available at: www.merck.com/newsroom/press_releases/research_and_development. Accessed September 12, 2007.
21. Briefing document (background package) from Merck for FDA Antiviral Drugs Advisory Committee Meeting September 5, 2007. Available at: www.fda.gov/ohrms/dockets/ ac/07/
briefing/2007-4314b1-01-Merck.pdf. Accessed September 11, 2007.
22. DeJesus E, Berger D, Markowitz M, et al. Antiviral activity, pharmacokinetics, and dose response of the HIV-1 integrase inhibitor GS-9137 (JTK-303) in treatment-naïve and treatment-experienced patients. J Acquir Immune Defic Syndr. 2006;43:1-5.
23. Zolopa AR, Mullen M, Berger D, et al. The HIV integrase inhibitor GS-9137 demonstrates potent antiretroviral activity in treatment-experienced patients. Program and abstracts of the 14th Conference on Retroviruses and Opportunistic Infections; February 25–28, 2007; Los Angeles, CA. Abstract 143LB.
24. Hosein SR. Anti-HIV agents. Raltegravir--other issues. TreatmentUpdate. 2007;19:9-10.
25. Pommier Y, Johnson AA, Marchand C. Integrase inhibitors to treat HIV/AIDS. Nat Rev Drug Discov. 2005;4:236-248.
26. Panacos Pharmaceuticals. Bevirimat (PA-457). Available at: www.panacos.com/
product_2.htm. Accessed July 9, 2007.
27. Beatty G, Lalezari J, Eron J. Safety and antiviral activity of PA-457, the first-in-class maturation inhibitor, in a 10-day monotherapy study in HIV-1 infected patients. Abstracts of the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy; December 16-19, 2005; Washington, DC. Abstract H-416 d.





