US Pharm. 2020;45(2):48-CV3.
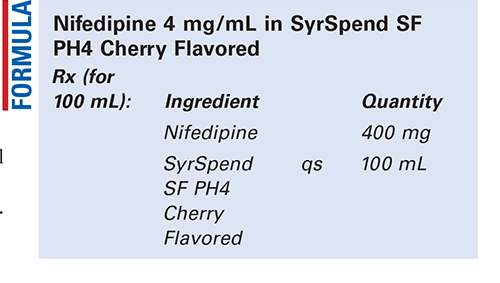
Method of Preparation: Note—Work efficiently and in subdued lighting, as nifedipine is highly sensitive to light.
Calculate the required quantity of each ingredient for the total amount to be prepared. Accurately weigh or measure each ingredient. Pulverize the nifedipine to a fine powder. Add a small portion of Syr-Spend SF PH4 Cherry Flavored to the powder and mix well to form a smooth paste. Geometrically, add additional SyrSpend SF PH4 Cherry Flavored to final volume in portions, and mix well after each portion. Package and label.
Use: Nifedipine is used in the management of Prinzmetal’s variant angina and chronic stable angina pectoris, hypertension, Raynaud’s phenomenon, preterm labor, and acute myocardial infarction.
Packaging: Package in tight, light-resistant containers.1
Labeling: Keep out of reach of children. Use as directed. Keep at room temperature or refrigerated. Shake well. Discard after ____ [time period].
Stability: A beyond-use date of 92 days may be used for this preparation when it is stored at room temperature or refrigerated temperature and packaged in low-actinic plastic prescription bottles. At the end of a 92-day stability study, 94.03% of the drug remained at room temperature and 90.83% remained at refrigerated temperature.2
Quality Control: Quality-control assessment can include weight/volume, pH, specific gravity, active drug assay, color, rheologic properties/pourability, physical observation, and physical stability (discoloration, foreign materials, gas formation, mold growth).3
Discussion: Nifedipine (Adalat, Procardia, C17H18N2O6, MW 346.33) is a 1,4-dihydropyridine-derivative calcium-channel blocking agent used in the management of Prinzmetal’s variant angina and chronic stable angina pectoris, hypertension, Raynaud’s phenomenon, preterm labor, and acute myocardial infarction. It occurs as a yellow powder that is affected by exposure to light. Nifedipine is practically insoluble in water and soluble in alcohol. Nifedipine extended-release tablets also contain black iron oxide; cellulose acetate; colloidal silicon dioxide; hypromellose; lactose monohydrate; magnesium stearate; microcrystalline cellulose; polyethylene glycol; polyethylene oxide; polysorbate; povidone; propylene glycol; red ferric oxide; sodium chloride; titanium dioxide; and triacetin. Procardia capsules are formulated as soft gelatin capsules for oral administration, each containing nifedipine 10 mg. Inert ingredients in the Procardia formulation include glycerin; peppermint oil; polyethylene glycol; soft gelatin capsules (which contain Yellow 6 and may contain Red Ferric Oxide and other inert ingredients); and water. The 10-mg capsules also contain saccharin sodium.4
SyrSpend SF PH4 is available as a liquid or as a dry powder for reconstitution. It is available in both liquid and powder form. The liquid is preserved with less than 0.1% sodium benzoate and is buffered to a pH of 4.2. SyrSpend SF PH4 is available either unflavored or as a cherry-flavored vehicle. The powder for reconstitution is preservative free, is buffered to a pH of 4.2, and is available as a preweighed powder to make 100 mL (containing 6.5 g powder) or 200 mL (containing 13.0 g powder). It is unflavored.5
It is important to note that this formulation in the published stability study used SyrSpend SF PH4 Cherry Flavored, so the beyond-use date applies to this preparation. This is important because SyrSpend products also include SyrSpend SF Alka Cherry (Preservative Free); SyrSpend SF Alka Unflavored (Preservative Free); SyrSpend SF Cherry; SyrSpend SF Grape; SyrSpend SF PH4 Dry (Preservative Free); SyrSpend SF Powder Dry (Preservative Free); and SyrSpend SF Unflavored.
SyrSpend products are oral starch-based suspension vehicles containing a patented Active Suspending Technology that holds active pharmaceutical ingredients’ particles in suspension and accelerates redistribution of suspended medication for more accurate dosing. They are buffered to a pH of 4.2 and have low osmolality, along with bitterness masking. SyrSpend products are formulated without alcohol, parabens, sorbitol, carrageenan, sugars, gluten, dyes, glycerin, propylene glycol, or benzyl alcohol. The SyrSpend SF family of products available in powder form require reconstitution with purified water prior to use. The SyrSpend SF Alka vehicles are buffered to a pH of >7. SyrSpend SF Alka is an easy-to-use powder for reconstitution that provides an alkaline environment for acid-labile drugs.6
REFERENCES
1. U.S. Pharmacopeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; January 2020.
2. Geiger CM, Sorenson B, Whaley P. Stability assessment of 10 active pharmaceutical ingredients compounded in SyrSpend SF. IJPC. 2015;19(5):420-427.
3. Allen LV Jr. Standard operating procedure for performing physical quality assessment of oral and topical liquids. IJPC. 1999;3:146-147.
4. RxList. Procardia. www.rxlist.com/procardia-drug.htm. Accessed January 5, 2020.
5. SyrSpend SF. https://fagron.com/en/product-innovations/syrspend%C2%AE-sf. Accessed January 5, 2020.
6. SyrSpend® SF family of products. https://us.fagron.com/en-us/syrspend-sf-family-products. Accessed January 5, 2020.
To comment on this article, contact rdavidson@uspharmacist.com.





