US Pharm. 2019;44(7):16-19.
ABSTRACT: Obstructive sleep apnea (OSA) is a chronic condition in which the upper airway collapses frequently during sleep and leads to breathing cessation. Higher-risk populations include males, women who have reached menopause, obese individuals, and patients with craniofacial abnormalities. Persons with OSA present with snoring, choking during sleep, nocturia, and excessive daytime sleepiness. Failure to address OSA not only compromises the patient’s quality of life but also increases the risk of mortality. Pharmacists can assist in the management of OSA by providing counseling regarding therapy and recommending lifestyle changes.
Obstructive sleep apnea (OSA), a breathing disorder that occurs during sleep, is characterized by a partial or complete blockage of the upper airway. OSA is caused by the collapse of soft tissue and muscles in the upper airway between the hard plate and the larynx.1 The collapsible part of the airway can completely obstruct or partially block the upper respiratory tract, leading to hypopnea and hypoxemia.1 The brain responds to the decrease in oxygen by arousing the body, causing brief wakefulness in order to restore oxygen concentration.1 Unfavorable upper-airway anatomy, i.e., a smaller mandible and enlarged tongue due to an inferiorly positioned hyoid bone, can contribute to oropharyngeal airway narrowing in OSA.2,3 In the obese population, fat deposits in the upper airway can cause narrowing, resulting in hypoxic events.4 Managing OSA early can increase quality of life and prevent complications.
Epidemiology
OSA has become an increasingly prevalent disorder, with current data from the United States and Europe showing that OSA affects between 14% to 49% of middle-aged men.5 OSA is markedly more prevalent in men than in women (24% vs. 9%, respectively).5 OSA with daytime drowsiness also occurred at a higher rate in men than in women (3%-7% vs. 2%-5%, respectively).6 Obesity is a leading risk factor for OSA, and the prevalence of this disorder in the obese population with a greater BMI is significantly higher.5,6 OSA is more common with increased age.7
Etiology
The pathogenesis of OSA is primarily attributed to abnormal pharyngeal anatomy, but medical conditions can also be a factor. Diseases associated with the risk of OSA include congestive heart failure, atrial fibrillation, hypertension, type 2 diabetes, nocturnal dysrhythmia, stroke, and pulmonary hypertension.8 Other characteristics that increase the probability of developing OSA include obesity, race (African Americans are at higher risk), smoking, and supine sleeping position.6,9 Certain medication classes can exacerbate OSA as well. These classes include opiates, benzodiazepines, myorelaxants, and drugs that have the side effect of weight gain (TABLE 1).10

Diagnosis
Persons at high risk for OSA should undergo objective testing via polysomnography (PSG) at a facility or by performing a home test with a portable monitor (PM).8 PSG (i.e., sleep study) can help detect anomalies in sleep patterns by recording physiologic signals such as airflow, oxygen saturation, respiratory effort, and heart rate.8 PMs also record parameters such as airflow, respiratory effort, and blood oxygenation but tend to be more limited than PSG.8 Although the main advantage of the PM is that it can be used in an unattended setting, it requires thorough evaluation by a trained clinician to ensure an accurate diagnosis.8
The American Academy of Sleep Medicine (AASM) published a clinical guideline on the selection of diagnostic tools that suggests that PSG or a PM be used for diagnosis in uncomplicated adults at increased risk for OSA.11 No other clinical tools are recommended. In patients with comorbidities that can potentially cause respiratory weakness (hypoventilation, chronic opioid user, stroke history, or severe insomnia), PSG is recommended over PMs because these conditions can undermine the PM’s accuracy.11,12 PMs are associated with significant false-negative rates—as high as 17%.12 If a single PM result is negative or deemed technically inadequate, PSG should be performed to rule out a false-negative result.12 Diagnosis based on PM results should be thoroughly evaluated by a professional board-certified in sleep medicine.8
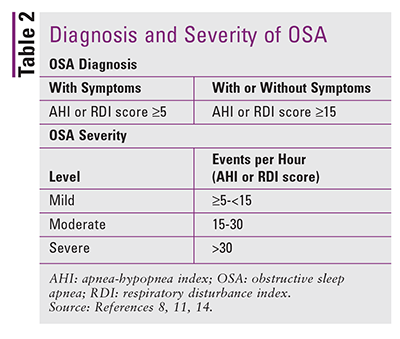
The two major indices reporting the frequency of obstructive events are the apnea-hypopnea index (AHI) and the respiratory disturbance index (RDI).8 The AHI, which is the average number of episodes of apnea and hypopnea per hour, is based on actual sleep (minimum duration of 2 hours) recorded by PSG. The AHI defines an apnea event as cessation of airflow for at least 10 seconds.8,13 Hypopnea is an abnormal respiratory event continuing ≥10 seconds with ≥30% reduction in thoracoabdominal movement or airflow compared with baseline, together with ≥4% oxygen desaturation.13 The AHI and RDI are often used interchangeably.13,14 The main difference is that the AHI also measures the number of respiratory effort–related arousals (RERAs) per hour. RERA refers to a series of respiratory cycles of increasing or decreasing effort that cannot be defined as apnea or hypopnea.15 Sleep stage and respiratory-event scoring are further specified in the AASM Manual for the Scoring of Sleep and Associated Events.16
The International Classification of Sleep Disorders (ICSD), a key reference for diagnoses of sleep disorders, is based on AASM manuals.14 The diagnosis of OSA requires symptoms coupled with five or more obstructive respiratory events (apneas, hypopneas, or RERAs) per hour of sleep as measured by PSG.14 Diagnosis may also be made if the frequency of obstructive respiratory events is 15 or more per hour regardless of symptoms.14 Whereas the ICSD uses the RDI as the index for obstructive respiratory events, the AASM considers the AHI the main index for scoring.11 OSA symptoms include daytime sleepiness, unrefreshing sleep, fatigue, insomnia, gasping or choking at night, loud snoring, and witnessed apneas.14 See TABLE 2.
Treatment
Two main clinical guidelines for the management of OSA have been developed by the American College of Physicians (ACP) and the AASM. The AASM published updated guidelines on specific OSA-management considerations in positive airway pressure (PAP) in 2019 and on oral-appliance therapy in 2015.17,18 The ACP guideline describes outcomes of studies that examined the efficacy and limitations of treatment options.19
PAP: Both guidelines agree that for all levels of severity in OSA, PAP is first-line therapy.8,17,19 PAP delivers compressed air to prevent airway closure, and its efficacy has been clinically proven in terms of reduced number of nocturnal obstructive events, reduced incidences of nocturnal arousals, and resolution of daytime symptoms after short-term use.20, 21 Modalities of PAP include continuous PAP (CPAP), bilevel PAP, and autotitrating PAP. CPAP, which delivers air at a fixed pressure, is usually administered through the nose.8,22 The most data exist on traditional CPAP, given that it has been in use longest.8 Studies demonstrated a significant reduction in crash risk of drivers with OSA following CPAP treatment.23 Health benefits of CPAP—e.g., lowering of blood pressure, decreases in cardiovascular events and coronary artery disease in adults with OSA, and improvement in lipid profile—have also been documented.24-26 Barriers to adherence include discomfort and claustrophobia.22,27
Oral Appliances: Oral appliances, such as the mandibular advanced splint (MAS)—also called mandibular repositioning appliance or mandibular advancement device (MAD)—are commonly used and may be an alternative to CPAP.8,19 Oral appliances are classified as positional therapy, wherein the mandible and its adjacent muscles are adjusted to widen the airway, thereby securing airflow.28 Studies show positive health outcomes such as decreased blood pressure and improved quality-of-life measures, especially with long-term treatment.29 Oral appliances require device adjustment over time. Other limitations in use include excessive salivation, dry mouth, and discomfort.29,30 Although MAS may be a feasible alternative to CPAP, evidence of benefit is lacking.19 The ACP states that MAD is an alternative treatment option for patients who fail or cannot tolerate CPAP therapy.19 The AASM guideline suggests that oral appliances may be selected over CPAP in mild-to-moderate disease based on patient preference or intolerance to CPAP.8,18 Tongue-retaining devices, which are designed to shift the tongue forward, have been associated with positive outcomes comparable to those for MAS.31
Behavioral Interventions: Behavioral therapy addresses lifestyle modifications to minimize risk factors for OSA. According to the AASM guideline, the main behavioral interventions for management of OSA include weight loss, exercise, positional sleep, and avoidance of alcohol, opiates, and sedatives before sleep.8 There is a linear relationship between obesity and OSA severity.19 Two studies—one of which assigned OSA patients to a low-energy, standardized, liquid-formulation diet for 9 weeks, and a randomized trial that put obese patients on a very-low-calorie diet along with other lifestyle modifications—demonstrated significant symptomatic improvement.32,33
Surgical Interventions: Surgery, which is invasive, is the last resort.19 The most common surgical intervention is uvulopalatopharyngoplasty (UPPP), which involves the removal of excessive tissue at the oropharyngeal tract, often accompanied by tonsillectomy.34 UPPP is shown to improve snoring and other sleep measures.34 Other procedures include palatal advancement, palatal implants, and tongue stabilization.8 Patients considering surgery should be assessed for eligibility based on specific anatomical features, psychological and social factors, and degree of willingness.8 Transient pain, difficulty swallowing, globus sensation, and voice change can occur.35
Adjunctive Therapies: The AASM guideline recommends adjunctive therapies, such as bariatric surgery and medications, in selected patients only.8 Bariatric surgery may be an option for those with a BMI ≥40 kg/m2, or with a BMI ≥35 kg/m2 with significant comorbidities.36 The ACP guideline points out that drug therapy was not shown to be effective in OSA management owing to inconsistent trial outcomes.19
The Pharmacist’s Role
Being a readily accessible healthcare professional, the pharmacist can identify risk factors for OSA. Early diagnosis can prevent further complications.37 A systematic review assessing the use of pharmacists in screening services for OSA showed that they were able to identify 21.4% to 67% of patients with OSA risk factors and refer them to a physician.37
The pharmacist can have a significant impact on optimizing CPAP management.38 Early involvement of healthcare providers in difficulties with CPAP therapy is associated with prolonged compliance.39 With patients new to therapy, the pharmacist can discuss patient understanding and concerns regarding CPAP use.
Counseling services may also include recommendations on lifestyle improvements such as weight loss and medication history. Avoidance of sedative-hypnotics and other drugs that can cause respiratory distress is imperative, especially in the setting of OSA treatment.37 The following are counseling points to discuss with patients with OSA40:
• Try sleeping on your side rather than on your back. This can help the throat stay open.
• Do not smoke or drink alcoholic beverages because they can worsen sleep apnea.
• Do not take any medications to help you sleep, as these products can worsen the symptoms of sleep apnea.
• If you are overweight, losing weight can help improve sleep apnea. It may decrease the amount of tissue that blocks your airway and let more air flow through.
Conclusion
OSA is a common condition involving a range of possible contributors, from structural anomalies to sleep habits. Failure to manage this disorder can lead to compromises in physical health as well as in daily performance. Therapy should be tailored to the individual patient and should be accompanied by regular follow-ups. Pharmacists can play an integral role in patient therapy by counseling patients, identifying barriers to therapy, and making referrals to primary care providers upon assessment.
REFERENCES
1. Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):144-153.
2. Chi L, Comyn FL, Mitra N, et al. Identification of craniofacial risk factors for obstructive sleep apnoea using three-dimensional MRI. Eur Respir J. 2011;38(2):348-358.
3. Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47-112.
4. Jehan S, Zizi F, Pandi-Perumal SR, et al. Obstructive sleep apnea and obesity: implications for public health. Sleep Med Disord. 2017;1(4):00019.
5. Garvey JF, Pengo MF, Drakatos P, Kent BD. Epidemiological aspects of obstructive sleep apnea. J Thorac Dis. 2015;7(5):920-929.
6. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136-143.
7. Kapur VK. Obstructive sleep apnea: diagnosis, epidemiology, and economics. Respir Care. 2010;55(9):1155-1167.
8. Epstein LJ, Kristo D, Strollo PJ Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
9. Menon A, Kumar M. Influence of body position on severity of obstructive sleep apnea: a systematic review. ISRN Otolaryngol. 2013;2013:670381.
10. Jullian-Desayes I, Revol B, Chareyre E, et al. Impact of concomitant medications on obstructive sleep apnoea. Br J Clin Pharmacol. 2017;83(4):688-708.
11. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479-504.
12. Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3(7):737-747.
13. Tunis S, Shuren J, Spencer FC, et al. Decision memo for continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea (OSA) (CAG-00093N). www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=19&fromdb=true. Accessed April 29, 2019.
14. Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387-1394.
15. Tsara V, Amfilochiou A, Papagrigorakis MJ, et al. Guidelines for diagnosis and treatment of sleep-related breathing disorders in adults and children. Definition and classification of sleep related breathing disorders in adults: different types and indications for sleep studies (Part 1). Hippokratia. 2009;13(3):187-191.
16. Berry RB, Brooks R, Gamaldo CE, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.3. Darien, IL: American Academy of Sleep Medicine; 2016.
17. Patil SP, Ayappa IA, Caples SM, et al. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2019;15(2):335-343.
18. Ramar K, Dort LC, Katz SG, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med. 2015;11(7):773-827.
19. Qaseem A, Holty JE, Owens DK, et al. Management of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159(7):471-483.
20. Patel SR, White DP, Malhotra A, et al. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163(5):565-571.
21. Stasche N. Selective indication for positive airway pressure (PAP) in sleep-related breathing disorders with obstruction. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2006;5:Doc06. Epub October 5, 2006.
22. Spicuzza L, Caruso D, Di Maria G. Obstructive sleep apnoea syndrome and its management. Ther Adv Chronic Dis. 2015;6(5):273-285.
23. Tregear S, Reston J, Schoelles K, Phillips B. Continuous positive airway pressure reduces risk of motor vehicle crash among drivers with obstructive sleep apnea: systematic review and meta-analysis. Sleep. 2010;33(10):1373-1380.
24. Montesi SB, Edwards BA, Malhotra A, Bakker JP. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med. 2012;8(5):587-596.
25. Milleron O, Pillière R, Foucher A, et al. Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study. Eur Heart J. 2004;25(9):728-734.
26. Nadeem R, Singh M, Nida M, et al. Effect of CPAP treatment for obstructive sleep apnea hypopnea syndrome on lipid profile: a meta-regression analysis. J Clin Sleep Med. 2014;10(12):1295-1302.
27. Chasens ER, Pack AI, Maislin G, et al. Claustrophobia and adherence to CPAP treatment. West J Nurs Res. 2005;27(3):307-321.
28. Chan AS, Sutherland K, Schwab RJ, et al. The effect of mandibular advancement on upper airway structure in obstructive sleep apnoea. Thorax. 2010;65(8):726-732.
29. Sutherland K, Vanderveken OM, Tsuda H, et al. Oral appliance treatment for obstructive sleep apnea: an update. J Clin Sleep Med. 2014;10(2):215-227.
30. Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29(2):244-262.
31. Randerath W, Verbraecken J, Andreas S, et al. Non-CPAP therapies in obstructive sleep apnoea. Eur Respir J. 2011;37(5):1000-1028.
32. Tuomilehto HP, Seppä JM, Partinen MM, et al. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2009;179(4):320-327.
33. Johansson K, Neovius M, Lagerros YT, et al. Effect of a very low energy diet on moderate and severe obstructive sleep apnoea in obese men: a randomised controlled trial. BMJ. 2009;339:b4609.
34. Verse T, Hörmann K. The surgical treatment of sleep-related upper airway obstruction. Dtsch Arztebl Int. 2011;108:216-221.
35. Franklin KA, Anttila H, Axelsson S, et al. Effects and side-effects of surgery for snoring and obstructive sleep apnea—a systematic review. Sleep. 2009;32(1):27-36.
36. Society of American Gastrointestinal and Endoscopic Surgeons. Guidelines for clinical application of laparoscopic bariatric surgery. www.sages.org/publications/guidelines/guidelines-for-clinical-application-of-laparoscopic-bariatric-surgery/. Accessed April 13, 2019.
37. Cawley MJ, Warning WJ II. A systematic review of pharmacists performing obstructive sleep apnea screening services. Int J Clin Pharm. 2016;38(4):752-760.
38. Shoukry G, Wong K, Bartlett D, Saini B. Treatment experience of people with obstructive sleep apnoea seeking continuous positive airways pressure device provision through community pharmacies—a role for pharmacists? Int J Pharm Pract. 2011;19:318-327.
39. Lewis KE, Seale L, Bartle IE, et al. Early predictors of CPAP use for the treatment of obstructive sleep apnea. Sleep. 2004;27:134-138.
40. Obstructive sleep apnea discharge instructions, adult. Patient education. Riverwoods, IL: Wolters Kluwer Health, Inc; Lexicomp. Accessed May 21, 2019.
To comment on this article, contact rdavidson@uspharmacist.com.





