US Pharm. 2020;45(3):HS-10.
ABSTRACT: The opioid-overuse crisis continues in the United States. Although many are familiar with the adverse events of opioid abuse, including respiratory depression, dependency, constipation, and hyperalgesia, not many may fully understand the effects of opioids on the immune system. The evidence supporting opioid effects on the immune system is vast: Chronic opioid abusers are potentially predisposed to higher rates of infection, including viral, bacterial, and fungal. Although the complete mechanism of the interaction between opioids and opioid receptors on the immune system is not fully elucidated, this review aims at furthering this discussion.
Overzealous opioid prescribing continues to be a national epidemic, leading to prescribing rates of 81.2 prescriptions per 100 persons in 2010, 33,091 deaths in the United States in 2015, and an estimated $78.5 billion spent annually on increased healthcare costs, substance-abuse treatment, and criminal justice costs.1 Although attempts to decrease opioid prescribing rates are ongoing, the opioid epidemic continues to be the worst drug crisis in U.S. history. Many healthcare providers recognize the common adverse events of opioids, including constipation, dependency, respiratory depression, hyperalgesia, and pruritus; however, the effect of opioids on the immune system remains underrecognized, with increased research efforts ongoing. An exhaustive overview of the immune system is beyond the scope of this review; however, it is imperative to recognize the differences in immunity prior to fully understanding the effects of opioids. This article aims to provide a summary of the effects of opioids on the immune system.
The immune system has two components—the innate and acquired immune system. The innate immune system functions as the first-line barrier and the most rapid response mechanism to prevent the invasion of microorganisms, whereas the adaptive immune system may take days to weeks to develop the appropriate responses of the T and B lymphocytes. Opioids act on the mu, delta, or kappa opioid receptors. Endorphin is the ligand for mu receptors and has a significant impact on analgesia, respiratory inhibition, and heart rate reduction. Enkephalin is the ligand for delta receptors and has no significant analgesic effect. Dynorphin is the ligand for kappa receptors and is responsible for analgesic properties, and it can induce anxiety with its weak respiratory inhibition effects.2 Previous studies have evaluated the opioid receptors on a wide array of immune cells. Exogenous and endogenous opioids can affect the innate and acquired immune systems and influence the immune response by interacting with the activation of different opioid receptors. Additionally, opioid peptides may be released from the nervous system with differing functionality on the immune system (TABLE 1).2-4 Although the complete mechanism of the interaction between opioids and opioid receptors on the immune system is not fully elucidated, this review aims at furthering this discussion.
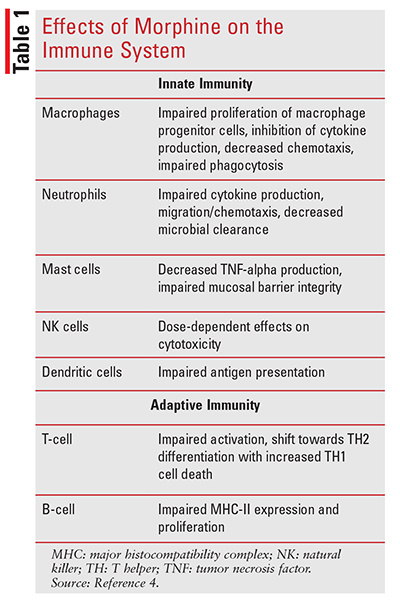
Effects of Opioids on Immune Cells
Opioids have considerable effects on cellular function across many immune cell lineages, affecting both the innate and adaptive immune systems. As mentioned, the innate immune system is our first-line defense against invading pathogens, and opioids, specifically morphine, have been shown to significantly impair various aspects of this response. Phagocytosis of invading pathogens by macrophages is a key component of this initial response. Morphine has been shown to negatively impact macrophage function by impairing the proliferation of macrophage progenitor cells, inhibiting cytokine secretion leading to decreased chemotaxis, hindering the ability of macrophages to directly phagocytize pathogens, and inhibiting nitric oxide production.5-9 Mechanistically, this is believed to occur through mu opioid receptors, as inhibition of phagocytosis was reversed in a study looking at mu opioid receptor knockout mice.10 The net result is impaired bacterial clearance by macrophages.
As with macrophages, opioids also impair cytokine production, migration, and microbial clearance capacity of neutrophils.4 Overall, the mechanisms of neutrophil inhibition by opioids are not as well elucidated as with macrophages; however, there is evidence that there is reduced interleukin (IL)-8 and keratinocyte-derived cytokine production, resulting in impaired chemotaxis.11,12 Dendritic cells are essential to the innate immune system through their presentation of foreign antigens to T-cells.13 Morphine has been shown to decrease IL-23 production in murine models, decreasing dendritic cell antimicrobial protein production.14 Relative to other cell lines, dendritic cells—in addition to mu receptors—have been shown to uniquely express delta opioid receptors in human models and kappa opioid receptors in murine models.15,16 Theoretically, this could manifest with opioids possessing varying receptor profiles to impact the immune system in different ways. Opioids also affect mast cells: studies have shown decreased TNF-alpha release as well as impaired mucosal barrier integrity, which is believed to be a result of TLR4-mediated negative crosstalk pathways. This may promote translocation of gut microflora, potentially increasing the risk of infection.4, 17-19
The final cell line of the innate immune system to be discussed is natural killer (NK) cells. Interestingly, the effect of morphine on NK cells seems to be dose-dependent: Lower doses of morphine (0.5 mg/kg in a porcine model) may increase the cytotoxicity of NK cells, while higher doses have been shown to decrease cytotoxicity. As a result, this has led researchers to believe that opioids’ effects on NK cells occur via an indirect mechanism.20-22
With regard to the adaptive immune system, opioids have been shown to affect T-cells and B-cells, both of which express mu opioid receptors.23 In murine models, morphine has been shown to decrease major histocompatibility complex (MHC)-II expression on B-cells, attenuating T-cell activation.19 Animal model studies have shown that opioids induce a plethora of molecular abnormalities within T-cells, including increased levels of cAMP, increased CREB/CREM/ICER activity, and increased IL-2 expression, while AP1, NFAT, NF-kB, c-fos, GATA-3 activity, and IFN-g are decreased.24-27 There is also an increase in TH1 apoptosis, resulting in a shift towards the TH2 phenotype, but it is not known what impact this has clinically.26
As previously discussed, the vast majority of the data investigating the immunosuppressive effects of opioids involves morphine.28 Only a few studies have investigated the comparative immunomodulatory effects of different opioids. In a study looking at animals undergoing surgery, impairment of NK cell activity was seen with fentanyl, morphine, and sufentanil.29 Interestingly, one murine comparative study demonstrated that morphine and oxycodone had immunosuppressive properties, while hydromorphone and codeine did not. The authors theorized that this is a result of differences in molecular structure, particularly substitution of a carboxyl group at C6, a single bond between the 7th and 8th carbon atoms, and the addition of a hydroxy group substituent to the molecule.30
Overall, the impact of opioids on the immune system is quite complex. Virtually all cell lines across both adaptive and innate immune systems are affected, weaving a complicated web that, as a whole, signals an impaired immune response, resulting in decreased pathogen clearance. While the preponderance of data stems from animal model studies evaluating the effects of morphine on a molecular level, it is nevertheless important to take the potential effects of opioids into consideration when utilizing these agents.
Implications of Opioid Impact on the Immune System
The evidence supporting opioids’ effect on the immune system is vast, with this impact potentially predisposing chronic opioid abusers to higher rates of infection, including viral, bacterial, and fungal. It is well documented that patients with opioid addiction have a higher risk of HIV infection.4 Opioids may promote the pathogenesis of HIV by modulating immune function or modifying the CNS response to HIV, as well as upregulating HIV entry into immune cells and enhancing activation and viral replication.4 Additionally, HIV opioid abusers are at higher risk for developing neurotoxicity and HIV-induced dementia. Hepatitis C virus (HCV) is also higher in those who abuse morphine, by enhancing HCV replication.
Opioid abusers also have higher likelihood of developing bacterial skin soft-tissue, respiratory tract, endovascular, and musculoskeletal infections.4 Increased susceptibility to respiratory infections, including Streptococcus pneumoniae, Staphlococcus aureus, Klebsiella pneumoniae, Mycobacterium, and Haemophilus, has been noted in those with chronic opioid abuse.4 Morphine has been shown to decrease bacterial clearance of S pneumoniae in macrophages by impairing TLR9-NF-KB signaling as well as dysfunction in IL-23 dendritic and macrophage cells with compromised neutrophil recruitment.4 Exposure of morphine in mice has been shown to increase the mortality with Candida albicans by decreasing phagocytosis by macrophages and polymorphonuclear leukocytosis.4
With ongoing knowledge of opioid abuse on the risk of opportunistic infections, a recent study aimed at comparing the 90-day mortality after ICU admission between chronic opioid users (³4 weeks) and opioid-naive patients.31 The authors found that the odds of 90-day mortality were higher in those with chronic opioid use than in opioid-naive patients, both in the generalized estimating equation model for propensity-matched cohort (odds ratio [OR] = 1.90; 95% CI, 1.57-2.31; P <.001) and in the multivariable logistic regression model for the entire cohort (OR = 2.20; 95% CI, 1.87-2.66; P <.001). The dose of opioid usage was calculated by converting to morphine equivalent daily dose (MEDD). The mean MEDD (SD) among chronic opioid users was 12.5 mg (21.0 mg).
Limitations of this study include the retrospective observational single-center design and the heterogeneity of critically ill patients from mixed ICUs.31 Additionally, this study was conducted in Korea, which may not be generalizable to the U.S. population given the MEDD. According to the CDC, in a national sample of Veterans Health Administration patients, those who died of opioid overdose were prescribed an average of 98 morphine milligram equivalents (MME) per day, while other patients were prescribed an average of 48 MME/day. Although this study had relatively low morphine equivalence, it still validates the concern for increased risk of mortality in those receiving chronic opioids.31
Additionally, a recent study evaluated the association of mortality in patients diagnosed with sepsis and the receipt of prescription opioids.32 Patients were considered opioid-treated if their medication administration record included opioid agents. Those exposed to opioids during hospitalization had a significantly higher 28-day mortality compared to those who did not receive opioids in the unadjusted and adjusted groups (hazard ratio [HR] 5.95, P <.001; HR 7.321, P <.001, respectively). Interestingly, opioid-treated patients had significantly higher gram-positive bacteria, gram-negative bacteria, and fungus on microbial culture data (39.32% vs. 20.43%, P <.001; 31.26% vs. 26.96%, P = .0019; 11.85% vs. 2.20%, P <.0001), which correlates with previous discussion of decrease of bacterial clearance.32 Overall, septic patients who were treated with opioids had a higher mortality at 28 days.32
Future Directions
As the opioid epidemic is ongoing, further research is needed to stratify the risk associated with prolonged opioid receipt on the immune system. Since IV opioid abusers are at high risk for developing bacterial endocarditis, it may be prudent to evaluate culture clearance data between non–IV opioid abusers. As a result of the interaction of opioids on the immune system, prolonged blood-culture clearance could be seen.
Pharmacist’s Role
Based on the current understanding of opioids on the immune system and continued research, it is reasonable to utilize opioid effects on the immune system in the repertoire of opioid stewardship. Pharmacists are encouraged to be opioid stewards in the inpatient and outpatient settings by understanding the potential effects on the immune system. Chronic opioid abusers not only have a high risk of overdose, but they also a high risk of septic mortality. Therefore, an additional review of opioid prescriptions in this population is warranted.
REFERENCES
1. Schuchat A, Houry D, Guy GP Jr. New data on opioid use and prescribing in the United States. JAMA. 2017;318(5):425-426.
2. Liang X, Liu R, Chen C, et al. Opioid system modulates the immune function: a review. Transl Perioper Pain Med. 2016;1(1):5-13.
3. Khademi H, Kamangar F, Brennan P, Malekzadeh. Opioid therapy and its side effects: a review. Arch Iran Med. 2016;19(12):870-876.
4. Roy S, Ninkovic J, Banerjee S, et al. Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections. J Neuroimmune Pharmacol. 2011;6(4):442-465.
5. Roy S, Wang J, Kelschenbach, et al. Modulation of immune function by morphine: implications for susceptibility to infection. J Neuroimmune Pharmacol. 2006;1(1):77-89.
6. Casellas AM, Guardiola H, Renaud FL. Inhibition by opioids of phagocytosis in peritoneal macrophages. Neuropeptides. 1991;18(1):35-40.
7. Szabo I, Rojavin M, Bussiere JL, et al. Suppression of peritoneal macrophage phagocytosis of Candida albicans by opioids. J Pharmcol Exp Ther. 1993;267(2):703-706.
8. Tomei EZ, Renaud FL. Effect of morphine on Fc-mediated phagocytosis by murine macrophages in vitro. J Neuroimmunol. 1997;74(1-2):111-116.
9. Menzebach A, Hirsch J, Nost R, et al. Morphine inhibits complement receptor expression, phagocytosis and oxidative burst by a nitric oxide dependent mechanism. Anasthesiol Intensivmed Notfallmed Schmerzther. 2004;39(4):204-211.
10. Roy S, Barke, RA, Loh HH. MU-opioid receptor-knockout mice: role of mu-opioid receptor in morphine mediated immune functions. Brain Res Mol Brain Res. 1998;61(1-2):190-194.
11. Glattard E, Welters ID, Lavaux T, et al. Endogenous morphine levels are increased in sepsis: a partial implication of neutrophils. PLoS One. 2010;5(1):e8791.
12. Martin JL, Koodie L, Krishnan AG, et al. Chronic morphine administration delays wound healing by inhibiting immune cell recruitment to the wound site. Am J Pathol. 2010;176(2):786-799.
13. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245-252.
14. Wang J, Ma J, Charboneau R, et al. Morphine inhibits murine dendritic cell IL-23 production by modulating toll-like receptor 2 and Nod2 signaling. J Biol Chem. 2011;286(12):10225-10232.
15. Makarenkova VP, Esche C, Kost NV, et al. Identification of delta- and mu-type opioid receptors on human and murine dendritic cells. J Neuroimmunol. 2001;117(1-2):68-77.
16. Kirst A, Wack C, Lutz WK, et al. Expression of functional kappa-opioid receptors on murine dendritic cells. Immunol Lett. 2002;84(1):41-48.
17. Meng J, Yu H, Ma J, et al. Morphine induces bacterial translocation in mice by compromising intestinal barrier function in a TLR-dependent manner. PLoS One. 2013;8(1):e54040.
18. Harari Y, Weisbrodt NW, Moody FG. The effect of morphine on mast cell–mediated mucosal permeability. Surgery. 2006;139(1):54-60.
19. Plein LM, Rittner HL. Opioids and the immune system—friend or foe. Br J Pharmacol. 2018;175(14):2717-2725.
20. Borman A, Ciepielewski Z, Wrona D, et al. Small doses of morphine can enhance NK cell cytotoxicity in pigs. Int Immunopharmacol. 2009;9(3):277-283.
21. Yokota T, Uehara K, Nomoto Y. Addition of noradrenaline to intrathecal morphine augments the postoperative suppression of natural killer cell activity. J Anesth. 2004;18(3):190-195.
22. Saurer TB, Carrigan KA, Ijames SG, Lysle DT. Suppression of natural killer cell activity by morphine is mediated by the nucleus accumbens shell. J Neuroimmunol. 2006;173(1-2):3-11.
23. Beagles K, Wellstein A, Bayer B. Systemic morphine administration suppresses genes involved in antigen presentation. Mol Pharmacol. 2004;65(2):437-442.
24. Wang J, Barke RA, Charboneau R, et al. Morphine negatively regulates interferon-gamma promoter activity in activated murine T cells through two distinct cyclic AMP-dependent pathways. J Biol Chem. 2003;278(39):37622-37631.
25. Borner C, Warnick B, Smida M, et al. Mechanisms of opioid-mediated inhibition of human T cell receptor signaling. J Immunol. 2009;183(2):882-889.
26. Roy S, Balasubramanian S, Sumandeep S, et al. Morphine directs T cells toward T(H2) differentiation. Surgery. 2001;130(2):304-309.
27. Roy S, Wang J, Gupta S, et al. Chronic morphine treatment differentiates T helper cells to Th2 effector cells by modulating transcription factors GATA 3 and T-bet. J Neuroimmunol. 2004;147(1-2):78-81.
28. Ninkovic J, Roy S. Role of the mu-opioid receptor in opioid modulation of immune function. Amino Acids. 2013;45(1):9-24.
29. Beilen B, Shavit Y, Hart J, et al. Effects of anesthesia based on large versus small doses of fentanyl on natural killer cytotoxicity in the perioperative period. Anesth Analg. 1996;82(3):492-497.
30. Sacerdote P, Manfredi B, Mantegazza P, Panerai AE. Antinociceptive and immunosuppressive effects of opiate drugs: a structure-related activity study. Br J Pharmacol. 1997;121(4):834-840.
31. Oh TK, Song IA, Lee JH, et al. Preadmission chronic opioid usage and its association with 90-day mortality in critically ill patients: a retrospective cohort study. Br J Anesthesia. 2019;122(6):e189-e197.
32. Zhang R, Meng J, Lian Q, et al. Prescription opioids are associated with higher mortality in patients diagnosed with sepsis: a retrospective cohort study using electronic medical records. PLoS One. 2018;13(1):e0190362.
To comment on this article, contact rdavidson@uspharmacist.com.





