US Pharm. 2024;49(6):HS-2-HS-10.
ABSTRACT: Advanced renal cell carcinoma (RCC), a metastatic kidney cancer, presents a significant challenge in modern oncology due to its increasing incidence and complex pathophysiology, with men being twice as likely as women to develop RCC. Recent advancements in treatment options, such as immune checkpoint inhibitors in combination with vascular endothelial growth factor receptor tyrosine kinase inhibitors, offer promising results in improving overall survival and progression-free survival rates for patients with advanced RCC. Despite the challenges posed by the high cost of therapies and medication shortages, pharmacists continue to play a key role in optimizing cancer care and ensuring positive treatment outcomes. Through collaboration with other healthcare professionals and advocacy for patients, pharmacists strive to enhance the overall quality of care and life for individuals battling advanced RCC.
Advanced renal cell carcinoma (RCC) is the most common type of metastatic kidney cancer, originating in the tubules within the kidney that aid in removing waste from the blood.1 RCC accounts for approximately 90% of all kidney cancer cases.2 The occurrence of kidney and renal pelvis cancers has significantly increased in the developed world in recent decades, with the incidence more than doubling in the United States since 1975. RCC is the seventh most common type of cancerous tumor in developed countries.3 Men experience double the risk of developing RCC compared with women. In the U.S., around 82,000 new cases of RCC and nearly 15,000 deaths are reported each year.4 Globally, kidney cancer contributes to over 400,000 new cases and more than 170,000 deaths annually.5
Pathophysiology
Advanced RCC is a diverse form of cancer that can arise from various cells across the nephrons. Numerous studies indicate that the histological subtype classification of RCC significantly influences both prognosis and treatment decisions. The classification of RCC into various subtypes by the World Health Organization is determined by its morphological, molecular, and genetic characteristics.6 Both clear cell carcinoma and papillary RCC stem from traits of the proximal tubule, while chromophobe RCC is derived from the distal connecting tubules and collecting duct system, specifically intercalated cells.7 Papillary RCC is a relatively slow-growing mass categorized into type I and type II, distinguished by histological and genetic differences. Routine imaging procedures cannot differentiate between these types. Both forms of papillary RCC—sporadic and hereditary—exist. Type I can be identified at an early stage compared with type II, resulting in a more favorable prognosis. Chromophobe RCC is more prevalent in the sixth decade of life.8
Risk Factors
Cigarette smoking, obesity, advanced age (55-74 years), and hypertension are widely acknowledged as the most firmly established risk factors for sporadic RCC. Acquired cystic kidney disease represents another notable risk factor.9 Risk factors with restricted or limited evidence include enhanced use of analgesics (acetaminophen and nonaspirin nonsteroidal anti-inflammatory drugs), diabetes mellitus, and exposure to specific chemicals in the workplace, such as trichloroethylene.10 Trichloroethylene is primarily recognized for its role as a solvent employed in the cleaning and degreasing of metal parts. Nonetheless, it has found diverse applications, such as an anesthetic, a heat-transfer medium, an extraction agent for fats and oils, an intermediate in the production of chlorofluorocarbons and various chemicals, and a component in numerous industrial and consumer products. In the U.S., trichloroethylene is accessible in various grades, including vapor-degreasing, general-purpose, and high-purity grades. Occupations that are readily exposed to this chemical are ones that involve metal cleaning and degreasing processes, manufacturing of electronics, machining industries, and dry-cleaning.11 Some minor risk factors may include increased triglycerides, end-stage renal disease, chronic kidney disease, and the enhanced intake of red and processed meat.12
Diagnosis
Diagnostic imaging methods, such as CT and MRI scans, can also disclose whether the cancer has metastasized to various parts of the body, such as the lungs, bones, liver, brain, or lymph nodes.1 Early detection of RCC is pivotal for effective treatment and lowering mortality rates in patients. Imaging techniques can identify renal masses with suitable accuracy. For instance, an abdominal ultrasound is frequently utilized for the detection of clinical benign cystic kidney lesions within RCC that can be readily diagnosed using an ultrasound, eliminating the necessity for further imaging assessments. Assessing the kidney function in a patient with RCC is crucial, as the treatment typically entails the complete or partial removal of a kidney.13 At diagnosis and follow-up visits, an assessment of the patient’s complete blood cell count, liver function tests, lactate dehydrogenase (LDH), calcium, alkaline phosphatase, urinary analysis, and other tests are crucial, depending on the appropriate treatment.14 For example, if patients receive tyrosine kinase inhibitor (TKI) therapy, such as axtinib, lenvatinib, or sunitinib, they should undergo thyroid function tests every 6 months. TKIs are often associated with thyroid dysfunction, specifically hypothyroidism, but they can also be linked to hyperthyroidism.15 Therefore, it is crucial that patients are monitored at baseline and at follow-ups for changes in their laboratory work or function tests.
Staging
Advanced RCC is categorized as stage 4. At this stage, the goal of therapy is usually to decelerate the advancement of the disease, mitigate symptoms, and enhance the overall quality of life (QoL). Nonetheless, ongoing research in advanced RCC is yielding new treatments that are gaining approval from the FDA.1
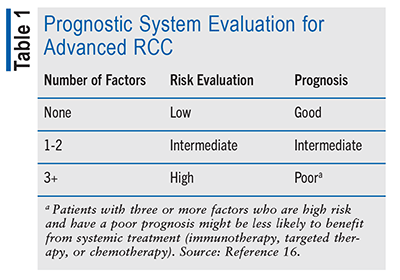
There are two prognostic systems that are currently used in determining needed treatments and forecasting outcomes in advanced RCC: Memorial Sloan Kettering Cancer Center (MSKCC) criteria and the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) criteria. In the MSKCC criteria system, five factors are used to evaluate which group the patient falls into (i.e., high-, intermediate-, or low-risk groups). These factors include high blood calcium level, anemia, high blood LDH level, poor performance status on daily tasks, and less than a year from diagnosis to the need for systemic treatment (chemotherapy, immunotherapy, or targeted therapy). The IMDC criteria include six factors: high platelet cell count, high white blood cell count (neutrophils), high blood calcium level, anemia, poor performance status on daily tasks, and less than a year from diagnosis to the need for systemic treatment (TABLE 1).16
Advanced RCC Treatment Algorithm Overview
The goals of therapy are to improve patients’ QoL and reduce disease progression. In patients with advanced RCC with an intermediate or poor prognosis and who are candidates for treatment with immune checkpoint inhibitors (ICIs), such as pembrolizumab, ipilimumab, or nivolumab, several treatment options are available. These patients may qualify for a first-line combination therapy of an ICI + ICI or ICI + vascular endothelial growth factor receptor tyrosine kinase inhibitor (VEGFR-TKI). Examples of a VEGFR-TKI include axitinib, cabozantinib, or lenvatinib. Patients with advanced RCC have five first-line combination therapy options to choose from: pembrolizumab + axitinib, nivolumab + cabozantinib, avelumab + axitinib, pembrolizumab + lenvatinib, or ipilimumab + nivolumab. The decision regarding treatment is made collaboratively between patients and their healthcare provider, taking into consideration various factors such as potential side effects, existing health conditions, financial considerations, and other relevant factors.17 The existing monotherapy treatments for advanced RCC have proven insufficient, resulting in low overall survival (OS) and progression-free survival (PFS) rates for patients. This inadequacy prompted the initiation of the KEYNOTE-426 clinical trial. This trial demonstrated that combination therapy led to significantly improved OS and PFS rates, ultimately enhancing patient health and survival outcomes.
KEYNOTE-426 Clinical Trial
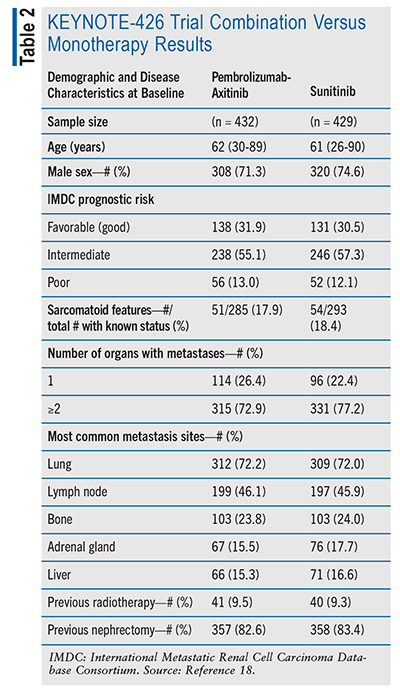
The KEYNOTE-426 trial was a randomized, open-label, phase III study to assess the efficacy and safety of combination therapy with pembrolizumab + axitinib versus monotherapy with sunitinib in first-line treatment for advanced RCC. This study primarily measured PFS and OS. This study had a secondary notable outcome measure of objective response rate (ORR). The PFS is the duration from randomization to the initial documented progressive disease or death from any cause, whichever happened first. The OS was characterized as the duration from randomization to death from any cause. The ORR was specified as the proportion of participants within the analysis population exhibiting a complete response (disappearance of all target lesions) or a partial response. Patients who were eligible for the study were aged 18 years or older and had either newly diagnosed or recurrent stage 4 RCC, had clear cell RCC, and had no history of receiving any previous systemic therapy for their advanced RCC.
Participants in the combination therapy arm received pembrolizumab + axitinib (n = 432) at baseline. In this treatment group, participants received an IV infusion of pembrolizumab 200 mg every 3 weeks and an oral dose of axitinib 5 mg twice daily. Participants in the monotherapy arm received sunitinib (n = 429) at baseline. In this monotherapy group, participants received oral sunitinib 50 mg once a day for 4 weeks and then were off treatment for 2 weeks. At baseline, there was a total of 861 participants. In the combination therapy arm, 28.7% (n = 124) of participants were female and 71.3% (n = 308) were male. In the monotherapy arm, 25.4% (n = 109) of participants were female and 74.6% (n = 320) were male. See TABLE 2.18
OS in Combination Versus Monotherapy
Regarding OS, 89.9% (95% CI, 86.4 to 92.4) of patients in the KEYNOTE-426 trial were alive at 12 months in the pembrolizumab-axitinib group and 78.3% (95% CI, 73.8 to 82.1) of patients were alive at 12 months in the sunitinib group. This represents a 11.6% decrease from the combined therapy treatment arm to the monotherapy arm.19
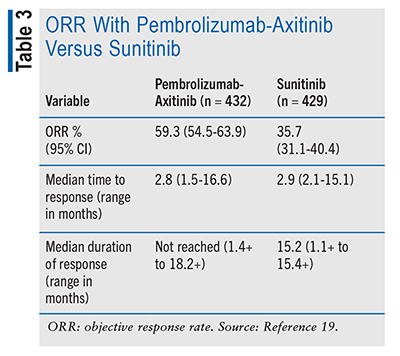
In the combination therapy arm, there was a complete ORR observed in 5.8% (n = 25) of participants, compared with the monotherapy arm, which saw a 1.9% (n = 8) complete response. A partial response was observed in 53.5% (n = 231) of participants in the combined therapy arm, while sunitinib had a 33.8% (n = 145) observed partial response rate.19
The combination regimen demonstrated a substantial enhancement in OS, PFS, and ORR compared with sunitinib monotherapy. These outcomes remained consistent across all IMDC subgroups, encompassing favorable-, intermediate-, and poor-risk groups. See TABLE 3.19
The Pharmacist’s Role
Pharmacists, especially those specializing in oncology, play a tremendous role in the comprehensive care of cancer patients throughout various stages of their treatment journey. This includes involvement in assessment and diagnosis, treatment-making decisions, medication management, addressing symptoms, providing supportive care, and participating in survivorship programs post treatment. Collaborating closely with other healthcare providers, pharmacists ensure the maintenance of accurate medication lists, selection of the most appropriate therapies, monitoring of medication efficacy, and management of adverse effects associated with cancer treatment. Chemotherapy agents are linked to various adverse effects, including but not limited to diarrhea, hypertension, reduced appetite, and nausea. It is important to monitor patients for serious adverse events, such as impaired thyroid function, proteinuria, thrombocytopenia, neutropenia, and anemia.19
Pharmacists also provide vital support to patients and their caregivers, offering guidance on medication regimens and anticipated side effects. Despite challenges such as the high cost of therapies, medication shortages, regulatory demands, and decreasing reimbursement, pharmacists remain essential members of the clinical team. Their contribution significantly enhances overall cancer care, thereby improving the QoL for patients.
There exist many opportunities for pharmacist involvement. Pharmacists can refer patients to copayment-assistance programs, giving them access to more cost-effective treatment alternatives. Pharmacists can also collaborate with other healthcare professionals to establish institutional guidelines and make evidence-based decisions aimed at enhancing patient care.20 Encouraging medication adherence by emphasizing the benefits and risks of therapy is paramount to achieving successful treatment outcomes. With the ability to address common patient complications such as nausea, pain, and depression, pharmacists serve as advocates for patients and their families, striving to enhance the quality of care and life of their patients.
Conclusion
Advanced RCC presents a significant healthcare challenge due to its increasing incidence and complexity. Despite advancements in understanding its pathophysiology and identifying risk factors, early detection is necessary for effective treatment and improved outcomes. Staging systems such as the MSKCC and IMDC criteria aid in prognosis and treatment decisions, guiding clinicians in tailoring therapies to individual patients. Clinical trials, such as KEYNOTE-426, have demonstrated the efficacy of combination therapies in improving survival rates and response rates compared with traditional monotherapy approaches.
Pharmacists, with their specialized knowledge and expertise, play a central role in the multidisciplinary care of RCC patients, contributing to treatment decision-making, medication management, symptom control, and supportive care. Despite challenges, pharmacists continue to advocate for optimal patient outcomes, striving to enhance the QoL for individuals who are affected by advanced RCC. Through collaboration with other healthcare professionals and the implementation of evidence-based practices, pharmacists contribute significantly to advancing cancer care and improving patient well-being.
REFERENCES
1. Kase AM, George DJ, Ramalingam S. Clear cell renal cell carcinoma: from biology to treatment. Cancers (Basel). 2023;15(3):665.
2. Hsieh JJ, Purdue MP, Signoretti S, et al. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3:17009.
3. Padala SA, Barsouk A, Thandra KC, et al. Epidemiology of renal cell carcinoma. World J Oncol. 2020;11(3):79-87.4. American Cancer Society. Cancer facts & figures 2023. Atlanta: American Cancer Society; 2023. www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2024-cancer-facts-figures.html. Accessed February 10, 2024.
5. Cirillo L, Innocenti S, Becherucci F. Global epidemiology of kidney cancer. Nephrol Dial Transplant. 2024. https://pubmed.ncbi.nlm.nih.gov/38341277. Accessed February 10, 2024.
6. Warren AY, Harrison D. WHO/ISUP classification, grading and pathological staging of renal cell carcinoma: standards and controversies. World J Urol. 2018;36(12):1913-1926.
7. Khoshdel Rad N, Vahidyeganeh M, Mohammadi M, et al. Non-clear cell renal cell carcinoma: molecular pathogenesis, innovative modeling, and targeted therapeutic approaches. Int J Transl Med. 2022;2(4):555-573.
8. Capitanio U, Bensalah K, Bex A, et al. Epidemiology of renal cell carcinoma. Eur Urol. 2019;75(1):74-84.
9. Kabaria R, Klaassen Z, Terris MK. Renal cell carcinoma: links and risks. Int J Nephrol Renovasc Dis. 2016;9:45-52.
10. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48.
11. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Trichloroethylene, tetrachloroethylene, and some other chlorinated agents. IARC Monogr Eval Carcinog Risks Hum. 2014;106:1-512.
12. Choueiri TK, Je Y, Cho E. Analgesic use and the risk of kidney cancer: a meta-analysis of epidemiologic studies. Int J Cancer. 2014;134(2):384-396.
13. Weng S, DiNatale RG, Silagy A, et al. The clinicopathologic and molecular landscape of clear cell papillary renal cell carcinoma: implications in diagnosis and management. Eur Urol. 2021;79(4):468-477.
14. Courcier J, de la Taille A, Nourieh M, et al. Carbonic anhydrase IX in renal cell carcinoma, implications for disease management. Int J Mol Sci. 2020;21(19):7146.
15. De Leo S, Trevisan M, Moneta C, Colombo C. Endocrine-related adverse conditions induced by tyrosine kinase inhibitors. Ann Endocrinol (Paris). 2023;84(3):374-381.
16. Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(1):71-90.
17. Singer EA, Rumble RB, Van Veldhuizen PJ. Management of metastatic clear cell renal cell carcinoma: ASCO guideline Q&A. JCO Oncol Pract. 2023;19(3):127-131.
18. Bedke J, Rini BI, Plimack ER, et al. Health-related quality of life analysis from KEYNOTE-426: pembrolizumab plus axitinib versus sunitinib for advanced renal cell carcinoma. Eur Urol. 2022;82(4):427-439.
19. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116-1127.
20. Mackler E, Segal EM, Muluneh B, et al. 2018 Hematology/Oncology Pharmacist Association Best Practices for the Management of Oral Oncolytic Therapy: Pharmacy Practice Standard. J Oncol Pract. 2019;15(4):e346-e355.
In memory of Dr. Essam Adly, who recently lost a 10-year battle with stage 4 advanced renal cell carcinoma.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






