US Pharm. 2022;47(7):34-40.
ABSTRACT: Coronavirus disease 19 (COVID-19) has been a global health crisis since late 2019. While vaccinations, monoclonal antibodies, IV remdesivir, and immunomodulatory therapies are now available to prevent disease and disease progression, there are limitations in their uptake or availability. The Emergency Use Authorization of two oral antiviral therapies, molnupiravir and ritonavir-boosted nirmatrelvir, in December 2021 greatly expanded outpatient access to therapeutics capable of preventing COVID-19 progression in high-risk patients diagnosed early in their disease course.
Infections due to the SARS-CoV-2 virus have led to a global pandemic, with over 500 million cases and more than 6 million deaths worldwide since late 2019.1 Initial recommended therapies such as dexamethasone, tocilizumab, and remdesivir focused on outcomes of reduction of mortality or reducing hospital length of stay. These therapeutics were primarily recommended for patients in the acute-care setting.2 Monoclonal antibodies have been developed for use in the outpatient setting as an IV infusion treatment option for patients at high risk for progression to hospitalization or death due to Coronavirus disease 19 (COVID-19). In December 2021, the FDA issued Emergency Use Authorizations (EUA) for two oral options for treatment of COVID-19 in the outpatient setting. The objective of this review is to compare safety, efficacy, and clinical data for molnupiravir and ritonavir-boosted nirmatrelvir (N-R).
Ritonavir-Boosted Nirmatrelvir (Paxlovid)
Overview and Development
Paxlovid is a combination of the drugs nirmatrelvir and ritonavir.3 Nirmatrelvir (formerly PF-07321332) is a novel SARS-CoV-2 peptidomimetic protease inhibitor active against Mpro. Mpro is a protease that contributes to viral replication by cleaving viral polyproteins. It may also be referred to as a 3CLpro or nsp5 protease inhibitor.3 Ritonavir has been utilized for decades as a protease inhibitor for the treatment of HIV.4 While ritonavir does not have activity against SARS-CoV-2, it is a strong CYP3A inhibitor that increases nirmatrelvir concentrations.3 The dosing of N-R is 300 mg of nirmatrelvir (two 150-mg tablets) with 100 mg (one 100-mg tablet) of ritonavir orally twice daily for 5 days. Patients should begin taking this medication as soon as possible after their diagnosis of COVID-19 and within 5 days of symptom onset.3
Dosing Adjustments
The dosing of N-R should be adjusted in renal dysfunction. In an open-label study evaluating its pharmacokinetics, the area under the curve of nirmatrelvir was increased by 87% in moderate renal impairment and 204% in severe renal impairment.3 For an eGFR of 30 to <60, the dosage should be reduced to 150 mg of nirmatrelvir with 100 mg of ritonavir taken twice daily. The packaging for N-R is unique, as it contains five blister packs, one for each day. For patients who require a dosage adjustment for renal function, pharmacists are required to remove the extra nirmatrelvir tablets and place a preprinted sticker over the empty blister areas. The Institute for Safe Medication Practices provides a detailed explanation of these instructions.5 In severe renal impairment with an eGFR <30, this medication is not recommended.3
No dosage adjustment is required for mild-to-moderate hepatic impairment. It is not recommended in severe hepatic impairment with Child-Pugh Class C.3
Efficacy
The primary clinical trial of N-R is the Evaluation of Protease Inhibition for Covid-19 in High-Risk Patients (EPIC-HR) trial.3,6 In this phase II/III multinational, double-blind trial, nonhospitalized symptomatic adults (N = 2,246) with a positive test for SARS-CoV-2 were randomized to receive N-R or placebo for 5 consecutive days. Patients were enrolled within 5 days of the onset of their symptoms and had a minimum of one COVID-19 sign or symptom on the day of randomization.6 To be included, they were required to have a minimum of one risk factor or characteristic for progression to severe disease. Patients were excluded if they had active liver disease, had moderate-to-severe renal impairment, were pregnant/breastfeeding, had known HIV with a viral load >400 copies/mL, or were taking medications such as ritonavir for HIV. Importantly, patients were also excluded if they were receiving medications that relied on CYP3A4 for clearance or medications that were strong CYP3A inducers. These patients had not received a vaccine for COVID-19 nor had a prior infection of COVID-19.6 Patients were permitted to receive the standard of care, including monoclonal antibodies, though the primary endpoint was only evaluated in those who did not receive a monoclonal antibody.
The primary endpoint in this study was hospitalization related to COVID-19 or death from any cause at 28 days.6 The average age was 46 years, and 51% of patients were men. The primary endpoint occurred in eight patients in the N-R group (0.77%) versus 66 patients in the placebo group (6.3%), which is an 88% relative risk reduction (P <.001). There were zero deaths in the N-R group and 12 in the placebo group. Adverse effects occurred in fewer than 10% of patients in each group. Adverse effects more common in the N-R group included dysgeusia and diarrhea.6
Interim data are also available for the Evaluation of Protease Inhibition for COVID-19 in Standard-Risk Patients (EPIC-SR) phase II/III trial.7,8 In this study, unvaccinated adults at standard risk and vaccinated adults with one or more risk factors for progressing to severe disease were randomized to receive N-R or placebo. This study had a unique primary outcome of self-reported resolution of symptoms for 4 consecutive days. Interim analysis demonstrated a nonsignificant relative risk reduction of 51%, and the study has ceased enrollment.7,8 N-R is also being evaluated for postexposure prophylaxis in the Epic PEP trial (currently ongoing).9
Clinical Application
N-R received EUA from the FDA on December 22, 2021.3 It is authorized for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients aged 12 years or older and weighing a minimum of 40 kg. Per the EUA, patients should have positive results of a SARS-CoV-2 viral test and be at high risk for progression to severe COVID-19, with potential for hospitalization or death.3 The CDC provides a list of underlying medical conditions associated with higher risk of severe COVID-19. These include cancer, cardiovascular disease, cerebrovascular disease, chronic kidney or liver disease, chronic lung conditions, diabetes, mental health disorders, obesity, smoking, and others.10 N-R demonstrates activity against all known human coronaviruses, including mutations such as the Omicron variant, though limited data are available.2,11 There are limitations to the EUA for this medication. This therapy is not authorized for initiation in the hospital setting, use as prophylaxis, or use for more than a 5-day duration.3 If a patient begins N-R as an outpatient and is then hospitalized, the 5-day course may be completed in the hospital setting.2
Per the National Institutes of Health (NIH) COVID-19 Treatment Guidelines, N-R is preferred (as an AIIa-level recommendation) over other outpatient treatment recommendations of remdesivir (BIIa), bebtelovimab (CIII), or molnupiravir (CIIa).2 However, there are no direct comparisons of efficacy or safety for these agents. Data are not available for combinations of these therapies.2 The Infectious Diseases Society of America suggests N-R within 5 days of symptom onset for outpatients with mild-to-moderate COVID-19 at high risk for progression to severe disease.12 The World Health Organization (WHO) recommends administering N-R in patients with nonsevere illness who are at the highest risk of hospitalization with a conditional recommendation against it in those who are at low risk for hospitalization.13
Special Populations
Though there are no human data for patients who are pregnant or breastfeeding, the NIH panel recommends N-R for pregnant patients as the benefits likely outweigh the risks.2 Additionally, the Society for Maternal-Fetal Medicine issued a statement supporting the use of N-R for the treatment of pregnant patients who meet clinical criteria. They state that it should not be withheld due to pregnancy or lactation.14 Though the EPIC-HR trial excluded pediatric patients, N-R received its EUA for pediatric patients aged 12 years or older and who weigh a minimum of 40 kg.3 The adult dose is expected to reach similar concentrations in these adolescent patients.3
Drug Interactions
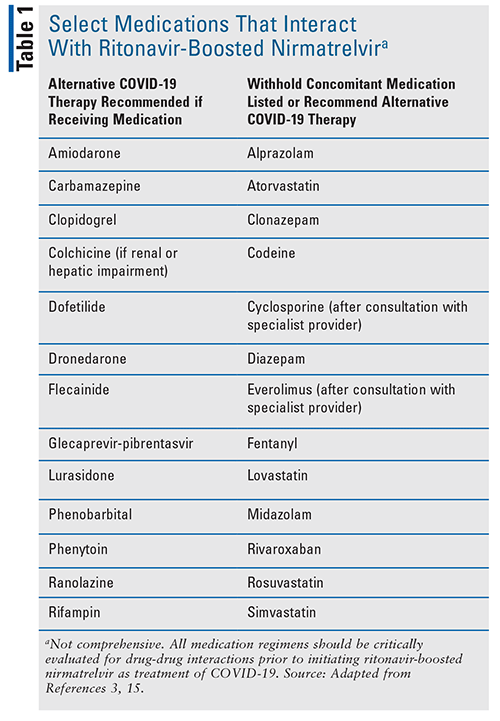
Pharmacists have a significant role in evaluating a patient’s medications for interactions with N-R. N-R is contraindicated with medications that are highly dependent on CYP3A for clearance or those that are strong inducers and could significantly reduce the concentrations of nirmatrelvir or ritonavir.3 The NIH recommends providers consult with a pharmacist or HIV specialist regarding the drug interactions with N-R.15 They also recommend obtaining a complete list of the patient’s medications, including those that are herbal and OTC. Strategies to facilitate the use of N-R include dose adjustment of the interacting medication (the dose of N-R should not be adjusted), use of an alternative of the interacting medication, increased monitoring, and/or temporarily holding the interacting medication.15 These strategies should be implemented for the 5-day course of N-R and for 3 to 5 days after completion of treatment.15 Patients should be informed of any of these drug interactions and if N-R can be safely used. A list of commonly prescribed medications and management strategies with N-R is provided (see TABLE 1), although this table is not comprehensive.15 Online databases and applications can be utilized to evaluate for drug-drug interactions, and the NIH guidelines recommend the use of the website https://www.covid19-druginteractions.org/ as a resource.15
Molnupiravir (Lagevrio)
Overview and Development
Molnupiravir is a broad-spectrum, oral antiviral agent that is a therapeutic option for adult outpatients with SARS-CoV-2 infection at high risk for progression to severe COVID-19.16 Molnupiravir is an oral, modified prodrug of N4-hydroxycitidine, which is a nucleoside analog that acts as an inhibitor of RNA-dependent RNA polymerase (RdRp).17 RdRp represents a critical step in a wide range of viruses in the viral replication transcription complex to copy viral RNA genome and create new virus. IV remdesivir also represents a nucleoside analog inhibitor of RdRp; however, the pathways by which viral replication is halted differs substantially between these two agents. While remdesivir acts as a competitive substrate in RNA genome that immediately halts the RNA replication process, molnupiravir triphosphate can be reused by SARS-CoV-2 as template strands for further viral RNA replication. This ultimately leads to an accumulation of copy-errors and a buildup of viral mutations that make the virus noninfectious and unable to replicate (“viral error catastrophe”).17
Molnupiravir represents a repurposed medication that was first developed in 2013 as a possible treatment for soldiers exposed to Venezuelan equine encephalitis virus, but it was found to have broad activity against a range of viral families, including influenza, Ebola, Chikungunya, respiratory syncytial virus, and coronaviruses.18 It was identified as a promising therapeutic option for SARS-CoV-2 soon after the COVID-19 pandemic began.18
Molnupiravir Dosing
Molnupiravir dosing is 800 mg (four 200-mg capsules) orally twice a day for 5 days.19 Similar to N-R, patients should begin taking this medication as soon as possible after their diagnosis of COVID-19 and within 5 days of the onset of symptoms.19 Unlike N-R, molnupiravir does not require any adjustments for renal or hepatic dysfunction, and it lacks any clinically relevant drug interactions.19
Molnupiravir Efficacy
Following initial phase I and phase II SARS-CoV-2 trials, molnupiravir was advanced into a phase III component of the MOVe-OUT trial in late 2020.20 This double-blind, randomized, placebo-controlled trial evaluated the effect of molnupiravir in nonhospitalized patients with COVID-19. The trial enrolled patients aged 18 years or older with confirmed SARS-CoV-2 infection and mild-to-moderate COVID-19 symptoms for no more than 5 days who had at least one high-risk factor for progression to severe disease (obesity, age over 60 years, diabetes, and severe cardiovascular disease, primarily). It is important to note that all patients enrolled in this trial were unvaccinated and that remdesivir and SARS-CoV-2 monoclonal antibody treatments were prohibited during this trial. Patients were randomized to receive molnupiravir 800 mg (four 200-mg capsules) or matching placebo orally twice daily for 5 days. The primary efficacy outcome was all-cause hospitalization or death through Day 29. Common features of the 1,433 patients in the final analysis included obesity (74% of patients) and a median age of 43 years. Of those enrolled, 48% had symptoms for fewer than 3 days, 55% had mild disease, 45% had moderate disease severity, and 20% had detectable SARS-CoV-2 nucleocapsid antibodies (indicating recent or previous infection).20
Hospitalization or death by Day 29 occurred in 6.8% of patients who received molnupiravir versus 9.7% of patients in the placebo group (treatment difference 3.0%, 95% CI -5.9 to -0.1). In a prespecified analysis focused only on hospitalizations or deaths likely to have been caused by COVID-19, the primary outcome occurred in 6.3% of molnupiravir-treated patients versus 9.2% of placebo-treated patients (risk difference 2.8%, 95% CI -5.7 to 0.0). Looking only at mortality, one patient (0.1%) in the molnupiravir group died, and nine patients (1.3%) in the placebo group died (89% decreased risk for death, 95% CI 14%-99%). In a time-to-event analysis, rate of hospitalization or death was 31% lower with molnupiravir than with placebo (hazard ratio 0.69, 95% CI 0.48-1.01). Subgroup analyses did not show any clear treatment benefit in those with undetectable SARS-CoV-2 or those with a positive SARS-CoV-2 antibody status at enrollment. No safety concerns were identified in the analysis of adverse events; however, it should be noted that pregnant patients were excluded in this study.20 Molnupiravir has been shown to have similar activity against the BA.1 and BA.2 variants as it did for more ancestral strains.21
Molnupiravir Clinical Application
Based on the results of the MOVe-OUT trial, an FDA advisory committee voted on November 30, 2021, by a narrow, 13-to-10 margin in favor of recommending it for EUA.22 Molnupiravir was granted EUA status by the FDA on December 23, 2021, and has been authorized for distribution in a number of other countries.16
While EUA status for molnupiravir has been granted, there are some important safety considerations for this agent based on its mechanism of action. First, molnupiravir has tested positive on the Ames test, indicating potential mutagenic potential in bacterial samples.18 While subsequent in vivo and in vitro analyses in rodent studies did not find evidence of genotoxicity (even at excessively high doses and durations), theoretical concerns for molnupiravir exist for use in pregnancy, children, and in males and females considering conception.18 As a result, molnupiravir should not be prescribed to pregnant or breastfeeding patients, and patients of childbearing potential should be counseled appropriately on intercourse around the time of molnupiravir administration.19 NIH guidelines advise patients to abstain from intercourse or use reliable contraception while taking molnupiravir, and for up to 4 days following the treatment course. These guidelines also advise that sexually active males of childbearing potential take similar precautions for 3 months following molnupiravir administration.2 Molnupiravir should not be used in patients younger than age 18 years due to potential risk for bone- and cartilage-growth abnormalities.19
A second consideration for molnupiravir is its theoretical risk for contributing to mutant variants of SARS-CoV-2.2,23 While any mutations caused by molnupiravir should eventually result in a mutant variant that is incapable of replication, any mutation generated by molnupiravir that resulted in a variant capable of escaping this viral death cycle would be of potential concern to global public health. As such, molnupiravir use should be avoided in patients who are unlikely to be able to adhere to a full 5-day course.2
Due to the relatively modest efficacy of molnupiravir observed in the MOVe-OUT trial, the potential safety concerns in pregnancy, and the theoretical potential for generation of novel SARS-CoV-2 variants, the NIH has advised that molnupiravir only be considered in select scenarios.2 The NIH recommends that nonhospitalized patients positive for SARS-CoV-2 at high risk for progression to severe COVID-19 be offered either N-R or IV remdesivir, and that molnupiravir should only be considered if both of these options are not available, or appropriate, for the patient. However, scaling of nirmatrelvir production may prove challenging to meet supply during another severe surge in cases, and outpatient infusions of IV medications can be logistically difficult, so there are patients who would still benefit from molnupiravir despite its limitations. Molnupiravir may also become the more readily available option in low- and middle-income countries.24 The WHO has given a conditional recommendation to consider molnupiravir in patients with nonsevere COVID-19 at highest risk for hospitalization, assuming strategies are in place to mitigate use in pregnant and breastfeeding women, as well as children.13
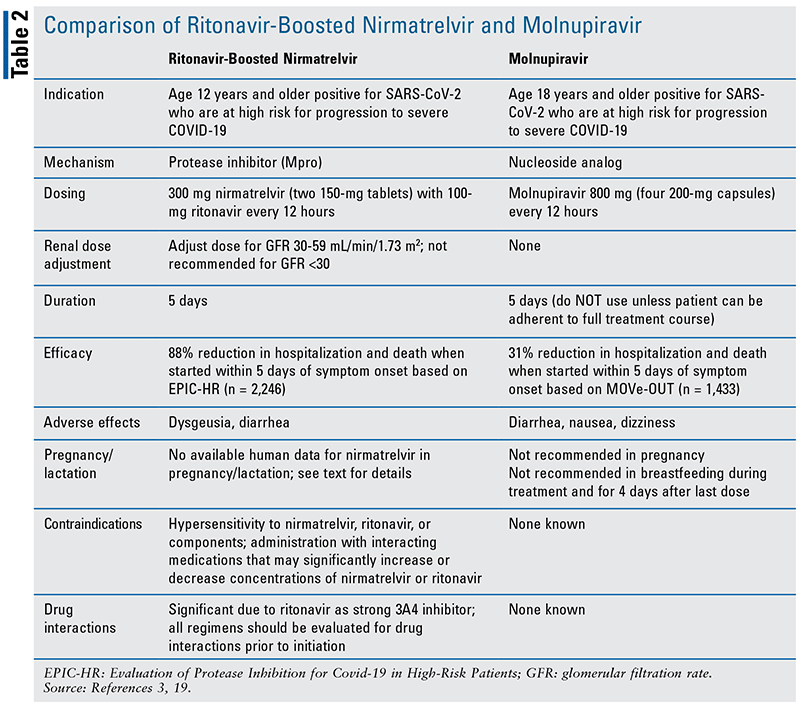
The emergence of two oral antivirals that have shown to be effective in the early stages of COVID-19 to prevent disease progression is an exciting event. A comparison of the characteristics of these agents can be found in TABLE 2. It allows practitioners to become even more multifaceted in how they prevent severe COVID-19 and associated deaths, adding to other effective methodologies, including vaccines, monoclonal antibodies, IV remdesivir, and immunosuppressive therapies, such as dexamethasone, tocilizumab, and baricitinib. While vaccines are widely available across the United States, the parenteral administration requirements for monoclonal antibodies and remdesivir have proven to be a substantial barrier to widespread adoption in the outpatient setting. The emergence and spread of the Omicron BA.1 SARS-CoV-2 variant and BA.2 subvariants in late 2021 have provided substantial challenges to healthcare systems in the U.S. and across the world. Their ability to escape immunomodulation from preexisting vaccines (particularly if not boosted) and monoclonal antibodies has further limited therapeutic options to mitigate severe disease. All of this makes the FDA EUA of N-R and molnupiravir very timely, relevant, and important. The rapid development and study of these medications highlights a high level of interprofessional ingenuity and teamwork necessary to accomplish this feat.
In 2021, the U.S. government committed to purchasing 3.1 million courses of molnupiravir, as well as 10 million courses of N-R.25,26 Similar to the SARS-CoV-2 vaccine rollout, doses are allocated directly to individual states, which distribute them accordingly. All doses allocated by the U.S. government will be available to dispense at no cost to patients; however, the acquisition cost is over $500 per treatment course, and out-of-pocket prices may be high once supply allocated by the U.S. government is exhausted.27 The U.S. Department of Health and Human Services offers a treatment locator tool online (https://covid-19-therapeutics-locator-dhhs.hub.arcgis.com/).
There are several limitations to these oral antiviral agents that should be considered by clinicians. Both were studied in patients who were unvaccinated against SARS-CoV-2 and in a time before the Omicron variants were present in these populations. As such, it is difficult to estimate the magnitude of benefit when prescribed to a vaccinated patient and in an era when a novel SARS-CoV-2 variant is dominant. Early analyses indicate, however, that both agents are likely to retain effective coverage against the Omicron BA.1 variant.28 Scaling the production and distribution of both oral medications will also be a challenge. Nirmatrelvir in particular has a complex chemical manufacturing process that limited its production capacity in the early months following FDA authorization, and this may be rate-limiting if a subsequent case surge ever causes a sharp spike in demand. Distribution is also variable from state to state, and in times of high demand, there will be a need to ensure that limited supplies are allocated to the patients at highest risk for disease progression (TABLE 3).
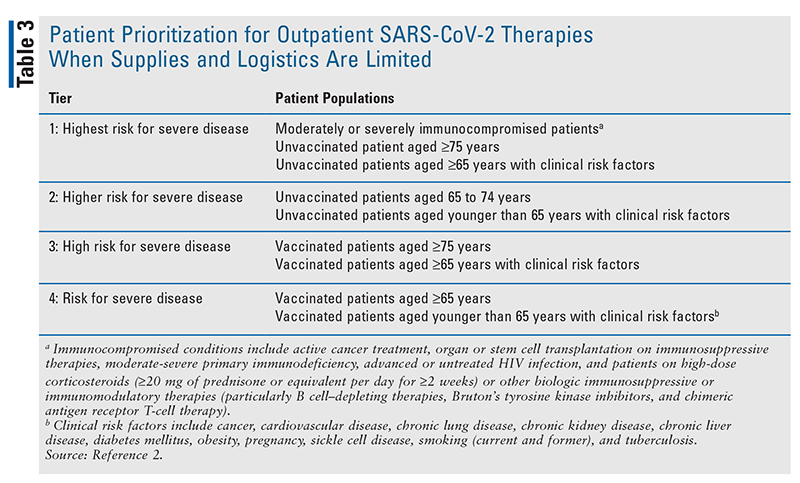
When supply of these medications is low, efforts should be made to ensure that patients at highest risk for COVID-19 disease progression be given highest priority. When medication supply is more ample, N-R should be the preferred oral antiviral for high-risk SARS-CoV-2 positive patients within 5 days of symptom onset given its superior efficacy in preventing hospitalization and death. Pharmacists must closely consider ritonavir-related drug interactions and be cognizant of dosing in renal impairment. When more efficacious treatment options are not available, molnupiravir should be considered as a viable, though less efficacious, alternative. Pharmacists and prescribers, however, must consider a number of factors when assessing candidacy for molnupiravir, including pregnancy and breastfeeding status, sexual partners in patients of childbearing potential, and the likelihood of medication adherence.
Conclusion
Despite limitations noted above, the development, authorization, and clinical availability of effective oral antivirals to prevent severe COVID-19 are important steps in combating this disease. There will be more research in the future to elucidate whether there is clinical benefit for N-R and molnupiravir in other situations (e.g., patients with acute infection without high-risk features, patients with long COVID-19 symptoms). Given that both molnupiravir and N-R have their own unique considerations, pharmacists will play an important role in patient selection, dosage adjustment, drug interaction management, dispensing, and patient counseling.
REFERENCES
1. World Health Organization. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/. Accessed April 28, 2022.
2. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. www.covid19treatmentguidelines.nih.gov/. Accessed April 21, 2022.
3. FDA. Fact sheet for healthcare providers: emergency use authorization for Paxlovid. www.fda.gov/media/155050/download. Accessed April 21, 2022.
4. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. Available at https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv. Accessed April 21, 2022.
5. Institute for Safe Medication Practices. Medication safety issues with newly authorized PAXLOVID. www.ismp.org/alerts/medication-safety-issues-newly-authorized-paxlovid. Accessed January 5, 2022.
6. Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386(15):1397-1408.
7. U.S. National Library of Medicine. Evaluation of protease inhibition for COVID-19 in standard-risk patients (EPIC-SR). https://clinicaltrials.gov/ct2/show/NCT05011513. Accessed January 13, 2022.
8. Pfizer Reports Additional Data on PAXLOVID™ Supporting Upcoming New Drug Application Submission to U.S. FDA. www.pfizer.com/news/press-release/press-release-detail/pfizer-reports-additional-data-paxlovidtm-supporting. Accessed June 14, 2022.
9. U.S. National Library of Medicine. A Study of a Potential Oral Treatment to Prevent COVID-19 in Adults Who Are Exposed to Household Member(s) With a Confirmed Symptomatic COVID-19 Infection. Available at https://clinicaltrials.gov/ct2/show/NCT05047601. Accessed April 21, 2022.
10. CDC. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare providers. www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html. Accessed January 5, 2022.
11. Hung YP, Lee JC, Chiu CW, et al. Oral nirmatrelvir/ritonavir therapy for COVID-19: the dawn in the dark? Antibiotics (Basel). 2022 Feb;11(2):220.
12. Bhimraj A, Morgan RL, Hirsch Shumaker A, et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. www.idsociety.org/globalassets/idsa/practice-guidelines/covid-19/treatment/idsa-covid-19-gl-tx-and-mgmt-v8.0.0.pdf. Accessed April 21, 2022.
13. World Health Organization. Therapeutics and COVID-19: living guideline. www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.3. Accessed April 24, 2022.
14. Society for Maternal-Fetal Medicine. SMFM supports the use of Paxlovid in pregnant patients. www.smfm.org/covidclinical. Accessed April 21, 2022.
15. National Institutes of Health. Ritonavir-boosted nirmatrelvir (Paxlovid). www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/ritonavir-boosted-nirmatrelvir--paxlovid. Accessed April 24, 2022.
16. O’Shaugnessy JA. Emergency use authorization letter of authorization. FDA. December 23, 2021. www.fda.gov/media/155053/download. Accessed January 13, 2022.
17. Malone B, Campbell EA. Molnupiravir: coding for catastrophe. Nat Struct Mol Biol. 2021;28(9):706-708.
18. Painter GR, Nathus MG, Cohen O, et al. Developing a direct acting, orally available antiviral agent in a pandemic: the evolution of molnupiravir as a potential treatment for COVID-19. Curr Opin Virol. 2021;50:17-22.
19. FDA. Fact sheet for healthcare providers: emergency use authorization for Molnupiravir. www.fda.gov/media/155054/download. Accessed January 13, 2022.
20. Bernal AJ, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386:509-520.
21. Takashita E, Kinoshita N, Yamayoshi S, et al. Efficacy of antiviral agents against the SARS-CoV-2 omicron subvariant BA.2 [letter to the editor]. N Engl J Med. 2022;386:1475-1477.
22. Final Summary Minutes of the Antimicrobial Drugs Advisory Committee Meeting November 30, 2021. FDA. www.fda.gov/media/154970/download. Accessed January 13, 2022.
23. Cully M. A tale of two antiviral targets—and the COVID-19 drugs that bind them. Nat Rev Drug Discov. 2022;21(1):3-5.
24. UNICEF. UNICEF enters supply agreements for COVID-19 oral antiviral medicine Molnupiravir. www.unicef.org/press-releases/unicef%20enters%20supply-agreements-covid-19-oral-antiviral-medicine-molnupiravir. Accessed January 21, 2022.
25. Merck and Co. Merck and Ridgeback Announce U.S. Government to purchase 1.4 million additional courses of molnupiravir, an investigational oral antiviral medicine, for the treatment of mild-to-moderate COVID-19 in at risk adults. www.merck.com/news/merck-and-ridgeback-announce-u-s-government-to-purchase-1-4-million-additional-courses-of-molnupiravir-an-investigational-oral-antiviral-medicine-for-the-treatment-of-mild-to-moderate-covid-19-in-a/. Accessed January 21, 2022.
26. Pfizer Inc. Pfizer to Provide U.S. Government with 10 million treatment courses of investigational oral antiviral xandidate to help combat COVID-19. www.pfizer.com/news/press-release/press-release-detail/pfizer-provide-us-government-10-million-treatment-courses. Accessed January 21, 2022.
27. Manas M. U.S. to buy 10 million courses of Pfizer’s COVID-19 pill for $5.3 billion. www.reuters.com/business/healthcare-pharmaceuticals/us-govt-buy-10-mln-courses-pfizers-covid-19-pill-529-bln-2021-11-18/. Accessed April 25, 2022.
28. Li P, Wang Y, Lavrijsen M, et al. SARS-CoV-2 Omicron variant is highly sensitive to molnupiravir, niramatrelvir, and the combination. Cell Res. 2022 Jan 10; Online ahead of print.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






