US Pharm. 2022;47(10):58-59.
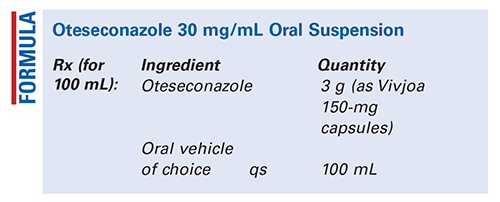
Method of Preparation: Calculate the required quantity of each ingredient for the total amount to be prepared. Accurately weigh or measure each ingredient. Empty the required number of capsules into a mortar and pulverize to a fine powder. Add a few mL of the vehicle of choice and mix to form a smooth paste. Geometrically, add sufficient vehicle of choice to final volume, mixing thoroughly after each addition. Transfer the preparation to a liquid dispensing bottle. Package and label.
Use: This preparation is an orally administered antifungal agent for those patients who have difficulty swallowing capsules.
Packaging: Package in tight, light-resistant containers.
Labeling: Keep out of reach of children. Discard after ____ [time period]. Shake well. Refrigerate.
Stability: The USP default beyond-use date for nonsterile oral liquids is 14 days when stored in a refrigerator; that should be appropriate for this preparation if a vehicle containing water is used.1
Quality Control: Quality-control assessment can include weight/volume, pH, specific gravity, active drug assay, color, rheologic properties/pourability, physical observation, physical stability (discoloration, foreign materials, gas formation, mold growth), and preservative-effectiveness test.2,3
Discussion: This formulation may be considered for patients who find it difficult to swallow intact capsules.
Vivjoa (oteseconazole), a new drug that was approved in April 2022, is an oral azole antifungal agent available in capsule form. The chemical name of oteseconazole is (R)-2-(2,4-difluorophenyl)-1,1-difluoro-3-(1H-tetrazol-1-yl)-1- (5-(4-(2,2,2-trifluoroethoxy)phenyl)pyridin-2-yl)propan-2-ol or 2-Pyridineethanol, α-(2,4- difluorophenyl)-β β-difluoro- α-(1H-tetrazol-1-ylmethyl)-5-(4-(2,2,2-trifluoroethoxy)phenyl)-,(αR)-. The drug’s empirical formula is C23H16F7N5O2. The molecular weight of oteseconazole is 527.39 g/mol.
Oteseconazole occurs as a white to off-white crystalline powder that is practically insoluble in water within a pH range of 1 to 9 but is soluble in a variety of organic solvents. Administration of oteseconazole with a high-fat, high-calorie meal (800-1,000 calories; 50% fat) increased maximum concentration of drug and AUC0-72h by 45% and 36%, respectively, but no significant differences were observed with a low-fat, low-calorie meal.4
Each oteseconazole capsule for oral use contains 150 mg of oteseconazole and the following inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, lactose, magnesium stearate, silicified microcrystalline cellulose, and sodium lauryl sulfate. The capsule shell and print constituents are FD&C Blue #1, FD&C Red #3, gelatin, Opacode SW-9008/SW-9009, and titanium dioxide. The capsules contain no ingredient made from a gluten-containing grain (wheat, barley, or rye).4
A number of appropriate official and commercial vehicles and suspending agents may be used for this preparation. These may be obtained from different companies, or they can be compounded. The following is a partial list of vehicles that may be considered for this preparation:
Cherry Syrup NF
Ora-Blend (Perrigo)
Ora-Blend SF (Perrigo)
Ora-Plus (Perrigo)
Ora-Sweet (Perrigo)
Ora-Sweet SF (Perrigo)
Oral Mix (Medisca)
Oral Mix, SF (Medisca)
Oral Syrup (Medisca)
Orange Syrup NF
PCCA Plus (PCCA)
Suspension Structured Vehicle NF
Suspension Structured Vehicle Sugar-Free NF
Vehicle for Oral Solution
Vehicle for Oral Solution, Sugar-Free
Vehicle for Oral Suspension
Because the active drug is insoluble in water and the other capsule excipients are water-insoluble, the final preparation will be a suspension. Therefore, a vehicle with appropriate suspending properties is needed. In addition, if bitterness is a problem, 0.2% stevia may be added to the final preparation.
REFERENCES
1. U.S. Pharmacopeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; September 2022.
2. Allen LV Jr. Summary of quality-control testing for sterile and nonsterile compounded preparations, part 1: physical and chemical testing. IJPC. 2019;23(3):211-216.
3. Allen LV Jr. Summary of quality-control testing for sterile and nonsterile compounded preparations, part 2: microbiological testing. IJPC. 2019;23(4):299-303.
4. RxList. Vivjoa. www.rxlist.com/vivjoa-drug.htm. Accessed September 26, 2022.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





