US Pharm. 2019;44(5)(Specialty&Oncology suppl):9-11.
ABSTRACT: Febrile neutropenia is a medical emergency that requires immediate evaluation and empirical antibiotics. Although many patients require hospitalization, some patients may be candidates for outpatient therapy. The 2018 joint guidelines of the American Society of Clinical Oncology and the Infectious Diseases Society of America provide guidance for the outpatient management of fever and neutropenia in adult patients treated for malignancy. Patients who are identified as low-risk based on clinical criteria and/or a validated scoring system can be treated empirically with an oral fluoroquinolone and amoxicillin-clavulanate. These patients should be continuously monitored and may need to be reevaluated for hospitalization.
Febrile neutropenia (FN) is a frequent, life-threatening complication in patients undergoing chemotherapy. The rate of major complications in patients diagnosed with FN is approximately 25% to 30%, with mortality as high as 11%.1,2 FN often leads to chemotherapy dosage reduction and treatment delays that may adversely affect long-term outcomes. Historically, the standard of care for all patients was inpatient management with IV antibiotics.3 However, some patients are now safely managed with oral antibiotics as outpatients. Advantages of treating patients with FN as outpatients include increased convenience, reduced costs of care, and reduced risk of healthcare-related infections.3,4 In 2018, the American Society of Clinical Oncology (ASCO) and the Infectious Diseases Society of America (IDSA) published joint guidelines updating their previous individual recommendations for the outpatient management of FN in adults treated for malignancy.1 Although assessment of risk and selection of empirical therapy remains with the physician, pharmacists should also be aware of current guidelines related to outpatient therapy in order to more effectively care for their patients.
Definitions and Pathogenesis
Neutropenia is defined as an absolute neutrophil count (ANC) of <1000 cells/µL, with severe neutropenia as an ANC of <500 cells/µL and profound neutropenia as <100 cells/µL. Fever in neutropenic patients is defined as a single oral temperature greater than or equal to 38.3°C (101°F) or a temperature of greater than or equal to 38.0°C (100.4°F) sustained over 1 hour. Fever may be the only indication of an underlying infection because signs and symptoms of inflammatory response are often diminished in these patients.1,5
The development of FN is believed to be due to damage to the gastrointestinal mucosal barrier, which can provide a portal of entry for endogenous flora, subsequent bacterial translocation, and possible infection.5 Patients receiving antineoplastics with a high incidence of mucositis are at increased risk. In addition, immune-system deficits associated with underlying malignancy and chemotherapy put patients at higher risk for infection. This risk increases as the ANC falls below 500 and in those patients with a prolonged duration of neutropenia.6
Initial Patient Management and Evaluation
Fever in neutropenic patients within 6 weeks of receiving chemotherapy is considered a medical emergency that requires a prompt evaluation by a medical professional and administration of empirical antibiotics.1,7 In the absence of an alternative explanation, clinicians should assume that fever in a patient with neutropenia from cancer therapy is the result of an infection.1 Initial patient evaluation should include a complete history and physical examination to identify the potential source of infection, CBC with differential, comprehensive metabolic profile, and at least two sets of blood cultures from different anatomic sources. Site-specific cultures and x-rays may be ordered as clinically indicated.1,4,5
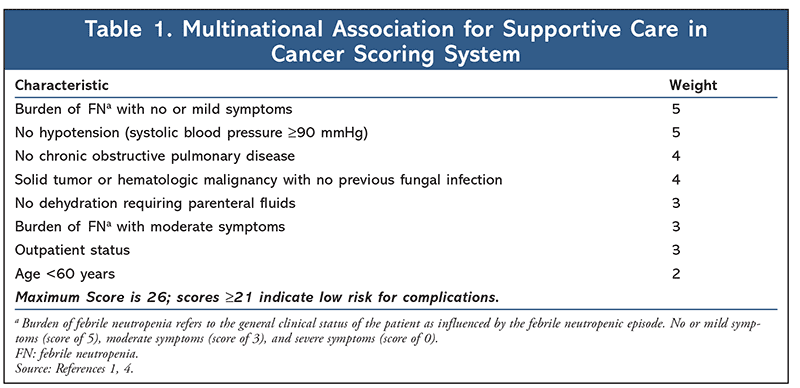
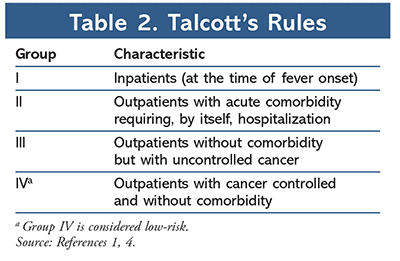
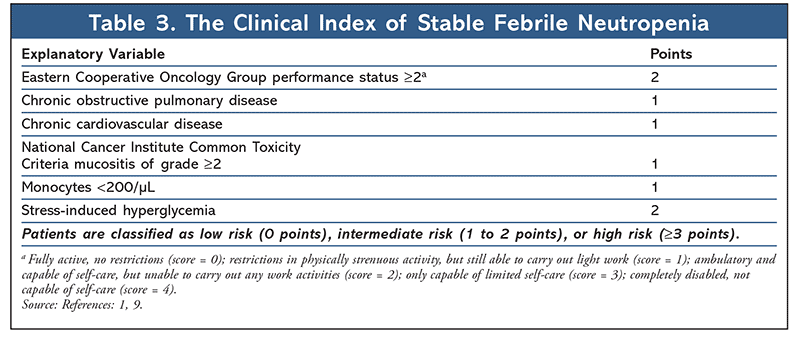
In order to identify appropriate patients for outpatient management, each patient presenting with FN must be assessed for risk of medical complications from severe infection. Patients deemed low-risk may be candidates for oral empirical therapy and outpatient management. The 2018 ASCO-IDSA guidelines recommend the use of clinical judgment when selecting candidates for outpatient management as well as algorithmic risk-assessment tools such as the Multinational Association for Supportive Care in Cancer (MASCC) index (Table 1) and Talcott’s Rules (Table 2).1,4 In addition, a more recently validated tool, the Clinical Index of Stable Febrile Neutropenia (CISNE), was recommended for predicting major complications in patients with seemingly stable FN (Table 3).1,8,9 Low-risk patients are typically those who are expected to be severely neutropenic (ANC <500 cells/µL) for less than 7 days, are clinically stable, have no comorbidities or evidence of organ dysfunction, and have a MASCC score greater than or equal to 21, a CISNE score of 0, or are classified as Talcott’s group IV. Further parameters that must be met to qualify for outpatient management include proximity to a clinic or hospital (less than or equal to 1 hour or less than or equal to 30 miles away), willingness to follow up frequently (daily for at least 3 days), 24-hour access to a caregiver, a telephone, transportation, and no history of noncompliance.1
Outpatient Treatment Recommendations
During the initial assessment and workup of FN, the patient should receive the first dose of empirical antibiotics while in the clinic, emergency department, or hospital. Empirical antibiotics should ideally be started after blood samples have been obtained and within 60 minutes of presentation. While some physicians may start low-risk patients on oral empirical antibiotics, others may initially prefer IV antibiotics before switching to an oral regimen. All patients should be observed for at least 4 hours prior to discharge in order to ensure that the fever is responding, they are clinically stable, and they can tolerate the prescribed antibiotics.1,4,5
The recommended oral empiric therapy for outpatient management consists of ciprofloxacin or levofloxacin plus amoxicillin-clavulanate. If the patient has a penicillin allergy, clindamycin may be substituted for amoxicillin-clavulanate.1 Fluoroquinolone monotherapy is not recommended, nor is outpatient management recommended if the patient received fluoroquinolone-based prophylaxis or if there is a high prevalence of extended-spectrum beta-lactamase–producing gram-negative bacilli, fluoroquinolone-resistant pathogens, or other resistant organisms (e.g., methicillin-resistant Staphylococcus aureus [MRSA], vancomycin-resistant enterococci).1,4 In patients with documented infection, the duration of therapy is dependent on the specific organism and site of infection. In patients without documented infection, antimicrobial treatment should at least continue for the duration of neutropenia (until ANC greater than or equal to 500 cells/µL).5
The recommended oral dose for ciprofloxacin is 750 mg twice daily, while levofloxacin is dosed at 500 mg or 750 mg by mouth once daily. Both medications have black box warnings for tendinitis and tendon rupture, peripheral neuropathy, central nervous system (CNS) effects, and myasthenia gravis exacerbations. Additionally, both have safety alerts for an increased incidence of aortic dissections or ruptures, significant hypoglycemia, and mental-health side effects. CNS and mental-health side effects include disorientation, memory impairment, delirium, seizures, psychiatric reactions, agitation, and nervousness. More common adverse effects include headache, insomnia, and gastrointestinal issues. Adverse effects may be increased in the elderly and dosage adjustments are needed in renal impairment. Fluoroquinolones may prolong the QTc interval, so concurrent use with other medications that affect the QTc interval or use in patients with a history of QTc prolongation should be avoided if possible. These medications must be taken at least 2 hours before or 6 hours after magnesium/aluminum antacids, sucralfate, chewable/buffered tablets, or other products containing calcium, iron, or zinc to avoid significant decreases in absorption.10
Amoxicillin-clavulanate is dosed at 500-125 mg orally every 8 hours or 1,000-250 mg every 12 hours. It is commonly associated with adverse gastrointestinal reactions, particularly diarrhea, but can also include nausea and vomiting. Allergic reactions (e.g., anaphylaxis, angioedema, rash) have been reported. Although not common, Clostridioides (formerly Clostridium) difficile–associated diarrhea (CDAD) and pseudomembranous colitis can occur. Absorption of clavulanate potassium is enhanced when administered at the start of a meal, which can also minimize the potential for adverse reactions and improve adherence.10
Clindamycin, if used owing to penicillin allergy, is dosed at 300 mg by mouth four times daily. Capsules should be taken with a full glass of water to avoid esophageal irritation. There is a black box warning for the risk of CDAD associated with clindamycin use, and this serious complication should be considered in all patients who experience diarrhea. Furthermore, pharmacists and patients should be aware that CDAD has been reported up to 2 months following treatment with clindamycin.10
Monitoring
In addition to the administration of appropriate empirical oral antibiotics, patients receiving outpatient therapy should be continuously monitoring their temperature for signs and symptoms of infection. Reliable temperature monitoring is essential, with oral thermometers recommended unless the patient has mucositis. If mucositis is present, then tympanic or axillary routes can be used, but the patient should be made aware that these may not be as reliable.6 Patients should expect to defervesce within 2 to 3 days of starting appropriate therapy and should be reevaluated and considered for hospitalization if 1) they do not defervesce; 2) if fever recurs after a period of defervescence; 3) there are new manifestations of infection; 4) the oral medication is no longer tolerated; or 5) a change in antimicrobial regimen is needed.1
Pharmacist’s Role
With their extensive pharmacotherapy knowledge, pharmacists have the opportunity to help optimize patient outcomes as part of the healthcare team. Pharmacists can make an impact on the outpatient treatment of FN by helping identify patients who can be managed on oral antibiotic therapy, selecting appropriate agents, monitoring treatment, and contributing to the development of guidelines for their institutions.11 Pharmacists may also provide detailed patient education, including the adverse effects of therapy, correct storage, appropriate administration, and the importance of adherence and self-monitoring. In addition, pharmacists should ensure that patients and caregivers are able to recognize signs and symptoms of infection as well as when to seek immediate evaluation and treatment.1,4
Conclusion
Successful treatment requires prompt patient evaluation, appropriate empirical antimicrobial therapy, and frequent patient monitoring. Patients with FN at low risk for complications can be managed effectively as outpatients. This therapy is preferred, when possible, because of convenience, lower cost, and decreased risk of hospital-related complications.
REFERENCES
1. Taplitz RA, Kennedy EB, Bow EJ, et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America clinical practice guideline update. J Clin Oncol. 2018;36:1443-1453.
2. Kuderer NM, Dale DC, Crawford J, et al. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106:2258-2266.
3. Teuffel O, Ethier MC, Alibhai SM, et al. Outpatient management of cancer patients with febrile neutropenia: a systematic review and meta-analysis. Ann Oncol. 2010;22:2358-2365.
4. Flowers CR, Seidenfeld J, Bow EJ, et al. Antimicrobial prophylaxis and outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31:794-810.
5. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e56-93.
6. Bow E, Marr KA, Thorner AR. Overview of neutropenic fever syndromes. In: Post TW ed. UpToDate. Waltham, MA: UpToDate; 2019. www.uptodate.com. Accessed February 4, 2019.
7. Brown CH. Febrile neutropenia infections. US Pharm. 2013;38(5):3-7.
8. Carmona-Bayonas A, Jiménez-Fonseca P, Virizuela Echaburu J, et al. Prediction of serious complications in patients with seemingly stable febrile neutropenia: validation of the Clinical Index of Stable Febrile Neutropenia in a prospective cohort of patients from the FINITE study. J Clin Oncol. 2015;33:465-471.
9. Oken, M, Creech R, Tormey D, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-655.
10. Lexicomp Online [online database]. Hudson, OH: Wolters Kluwer Health, Inc; 2019. http://online.lexi.com. Accessed February 16, 2019.
11. Pherwani N, Ghayad JM, Holle LM, Karpiuk EL. Outpatient management of febrile neutropenia associated with cancer chemotherapy: risk stratification and treatment review. Am J Health Syst Pharm. 2015;72:619-631.
To comment on this article, contact rdavidson at uspharmacist.com.