US Pharm. 2022;47(4):18-21.
ABSTRACT: COVID-19 represents a highly contagious virus that is pervasive and continues to evolve. Patients may present with a variety of symptoms, but most patients have mild illness and can be managed with supportive care. However, for outpatients at risk for disease progression, anti–SARS-CoV-2 treatment with an oral antiviral, monoclonal antibody, or remdesivir is currently recommended and significantly lowers the risk of hospitalization.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the novel virus responsible for the global COVID-19 pandemic. The first cases were reported in Wuhan, China, in December 2019 and have rapidly expanded across the globe.1-3 According to the World Health Organization, as of March 4, 2022, there have been more than 440 million cases reported worldwide with more than 5.9 million deaths.4
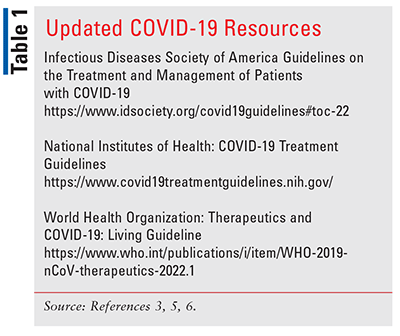
Although vaccines have made a significant impact by reducing risk of transmission and lessening severity of disease, COVID-19 remains widespread. Fortunately, >80% of cases are considered mild, so outpatient treatment is often appropriate.5 With recommendations continuing to change as clinical trials are completed and new therapies and variants emerge, pharmacists must be familiar with updated COVID-19 guidelines to provide the best care for patients (see TABLE 1).3,5,6
Anti–SARS-CoV-2 Treatment
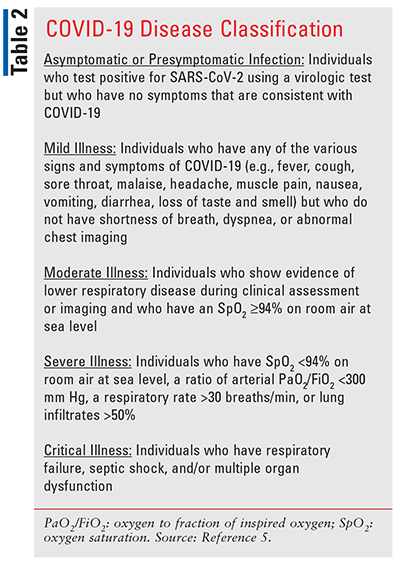
After a positive diagnosis, the decision to manage COVID-19 in the outpatient setting is largely based on severity of illness (see TABLE 2).5 Those with mild-to-moderate disease (defined as the absence of pneumonia and hypoxemia) can often be managed effectively with supportive care alone. However, symptomatic patients with certain underlying comorbidities (see TABLE 3) are at high risk of developing severe COVID-19 and should also receive anti–SARS CoV-2 therapy.6-8

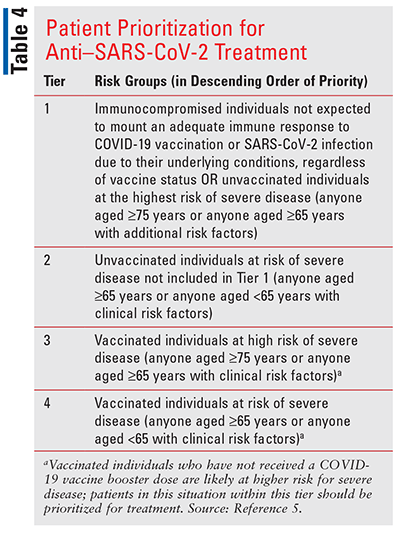
Current guidelines recommend nirmatrelvir/ritonavir (Paxlovid), sotrovimab (Xevudy), or remdesivir (Veklury). If these options cannot be used or are not available, molnupiravir (Lagevrio) or bebtelovimab may be used. These medications are not indicated for asymptomatic COVID-positive outpatients or those with mild-to-moderate disease without risk factors.3,5 The choice of therapy is dependent on clinical efficacy, susceptibility to prevalent variants, local availability, and patient-specific factors, including concomitant medications and comorbid conditions. Limited supplies may require further prioritization of treatment to those at highest risk (see TABLE 4).5
Antiviral Agents
In December 2021, the FDA issued an Emergency Use Authorization (EUA) for the new oral antivirals nirmatrelvir/ritonavir and molnupiravir for treatment of patients with mild-to-moderate COVID-19 who are within 5 days of symptom onset and at a high risk of progression to severe disease. They have not been authorized for pre-exposure or postexposure prophylaxis or for treatment of hospitalized patients due to severe COVID.9,10 Remdesivir is an IV antiviral that was approved by the FDA for patients requiring hospitalization and for nonhospitalized patients within 7 days of symptom onset with risk factors for progression.11
Nirmatrelvir/Ritonavir: Nirmatrelvir blocks the activity of the SARS-CoV-2 protease, an enzyme required for viral replication. Ritonavir, also a protease inhibitor and potent cytochrome P450 inhibitor, is utilized in this combination for its pharmacokinetic boosting ability, which provides higher concentrations of nirmatrelvir for prolonged periods. In a recent clinical trial, nirmatrelvir/ritonavir reduced the risk of hospitalization or death by 88% compared with placebo in nonhospitalized adults with COVID-19.9
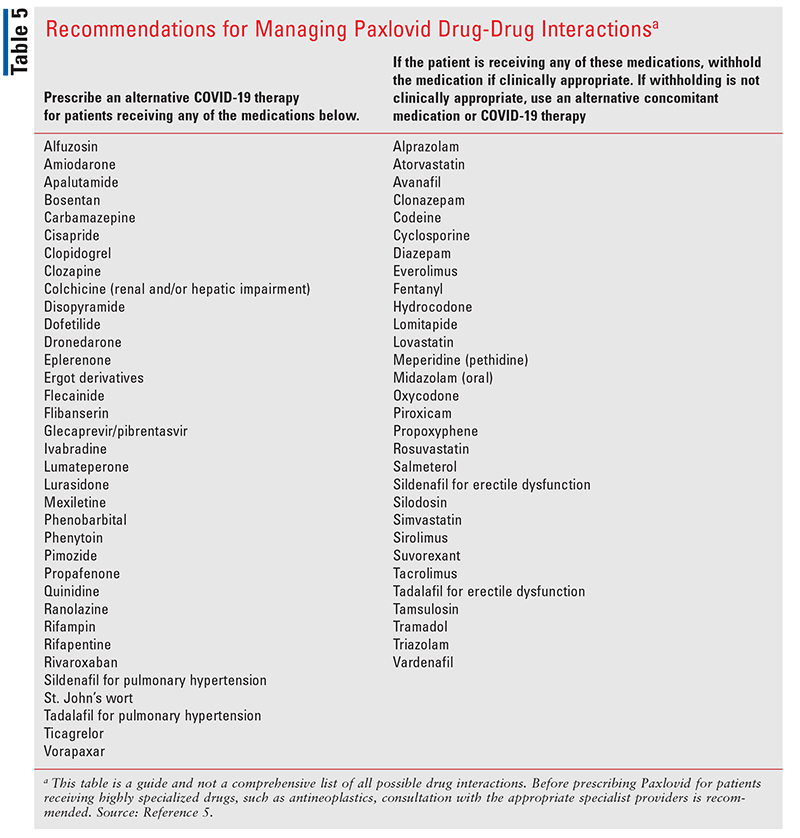
The dosage for patients with normal renal function is nirmatrelvir 300 mg (2 tablets) plus ritonavir 100 mg (1 tablet) orally twice daily for 5 days. In patients with compromised kidney function (glomerular filtration rate [GFR] 30-59 mL/min), the dosage should be lowered to 150 mg nirmatrelvir (1 tablet) plus ritonavir 100 mg (1 tablet) twice daily for 5 days. It is not recommended in patients with GFR <30 mL/min or with severe hepatic impairment. Side effects include impaired taste, diarrhea, increased blood pressure, and muscle aches.9,12 Nirmatrelvir/ritonavir is not an appropriate choice for patients taking certain medications, due to the potential for serious drug interactions (see TABLE 5).5,9
Molnupiravir: Molnupiravir interferes with the replication of SARS-CoV-2 by promoting widespread mutations. In a large, randomized, controlled trial, molnupiravir was found to reduce the risk of hospitalization by 31% in nonvaccinated patients with at least one risk factor for severe disease.13 Due to this lower rate of efficacy, it is considered an alternative option.3,5
The recommended dosage is 800 mg (4 200-mg capsules) orally twice daily for 5 days, and there is no dosage adjustment for renal or hepatic dysfunction. Side effects include diarrhea, nausea, and dizziness. No drug interactions have been identified based on available data. It is contraindicated in patients younger than age 18 years due to bone and cartilage toxicity and in pregnant or breastfeeding patients because of concerns regarding fetal toxicity. Pregnancy tests are recommended before starting this medication in women of childbearing age. All patients should be counseled on the importance of using a reliable method of contraception throughout treatment and for at least 4 days (females) or 3 months (males) after the last dose.10,12
Remdesivir: Remdesivir is a nucleotide analog that inhibits SARS-CoV-2 RNA–dependent RNA polymerase, which is essential for viral replication. In a recent study, remdesivir was found to decrease hospitalization by 87% compared with placebo in unvaccinated outpatients at risk for progression.14 These findings, as well as the prevalence of the Omicron variant and lack of efficacy among some monoclonal antibodies, prompted the addition of remdesivir to outpatient COVID guidelines as a treatment option in areas where Omicron was the predominant strain.3,5
Remdesivir requires parenteral administration over 3 days, which can make outpatient administration challenging. The recommended dosage is 200 mg on Day 1, followed by 100 mg on Days 2 and 3, and it is infused over 30 to 120 minutes. The manufacturer does not recommend use in patients with GFR <30 mL/min, and no dosage adjustment is necessary in hepatic failure. Adverse effects are minimal and similar to placebo. Remdesivir should not be used with hydroxychloroquine or chloroquine because they may diminish the therapeutic effect of remdesivir.11,12
Monoclonal Antibodies
In 2021, the anti–SARS-CoV-2 monoclonal antibodies bamlanivimab/etesevimab, casirivimab/imdevimab, and sotrovimab were issued EUAs by the FDA to treat symptomatic, COVID-positive outpatients at risk for severe disease. At that time, all three agents were found to be effective against circulating COVID-19 virus, including the Delta variant. However, subsequent mutations with the Omicron variant resulted in reduced susceptibility to bamlanivimab/etesevimab and casirivimab/imdevimab. This prompted the FDA to revise their authorization, limiting use to only susceptible variants. In February 2022, bebtelovimab was granted an EUA by the FDA. Of note, sotrovimab and bebtelovimab are currently the only monoclonal antibodies effective against the Omicron variant.5,15-18
Monoclonal antibodies should be given as soon as possible after a positive COVID test and within 7 days (bebtelovimab) or 10 days (sotrovimab, bamlanivimab/etesevimab, and casirivimab/imdevimab) of symptom onset. They require parenteral administration, and patients must be monitored for at least 1 hour after infusion. No dosage adjustments are needed in patients with renal or hepatic impairment. Adverse effects include infusion-related and hypersensitivity reactions, diarrhea, chills, and dizziness. Monoclonal antibodies may diminish the effectiveness of the COVID-19 vaccine, so patients should wait 90 days to get vaccinated or boosted.12,15-18
Sotrovimab: Sotrovimab is given as a single 500-mg IV infusion over 30 minutes.17
Bebtelovimab: The recommended dosage of bebtelovimab is 175 mg given as a single IV dose over at least 30 seconds.18
Casirivimab-Imdevimab: Casirivimab-imdevimab is available in single vials containing both antibodies or as individual antibody solutions in separate vials. The recommended dosage is 600 mg/600 mg and is given as a single IV infusion over 20 to 50 minutes. Subcutaneous injection is an acceptable alternative route of administration when IV administration is not available or would result in a delay of treatment. The single dose is divided into four subcutaneous injections using different quadrants of the abdomen, upper thigh, and back of the upper arms.16
Bamlanivimab/Etesevimab: The recommended dosage is bamlanivimab 700 mg and etesevimab 1,400 mg given as a single IV infusion. The antibodies are packaged in individual vials, so they must be mixed and further diluted with normal saline. The infusion is given over 21 to 70 minutes.15
Medications Not Recommended
Clinical guidelines currently recommend against the use of interferons, HIV protease inhibitors, colchicine, nitazoxanide, convalescent plasma, and hydroxychloroquine or chloroquine with or without azithromycin for outpatient treatment of COVID-19. Systemic glucocorticosteroids, antibiotics, anticoaguladnts, and/or antiplatelet therapy should also not be used to treat these patients in the absence of another indication. Ivermectin is not recommended due to insufficient evidence and lack of data from adequately powered, well-designed trials.5
Supportive Care
Supportive care for COVID-19 includes adequate hydration, proper nutrition, and plenty of rest. Antipyretics and analgesics can be recommended for patients complaining of fever, myalgias, and/or headaches. Cough that is persistent, interferes with sleep, or causes discomfort may be managed with OTC cough medications containing dextromethorphan or prescription benzonatate 100 to 200 mg by mouth three times daily as needed.5,12 Some patients may experience improvement with cough or dyspnea by resting in the prone position rather than in the supine position.5
Monitoring Parameters
Given the possible risk of deterioration, patients with risk factors for severe disease should be carefully monitored until they make a full recovery. Although not accessible to many patients, home pulse oximetry may assist in the earlier detection of severe disease. However, patients must have access to reliable oximeters and be adequately trained on how to use the machine, interpret the readings, and when to contact their provider. Counseling regarding signs and symptoms of worsening disease (such as dyspnea, dizziness, and mental status changes) and when to seek urgent care should be provided to all patients and their caregivers.5,6
Conclusion
Healthcare providers should identify COVID-positive patients who are at risk for progression to severe disease and promptly initiate appropriate anti–SARS-CoV-2 therapy. Given the ease of accessibility and extensive pharmacotherapy knowledge, pharmacists are uniquely positioned to help optimize patient outcomes by screening for major drug interactions, providing patient education (including appropriate administration and adherence, adverse effects of therapy, and storage requirements), monitoring patients for disease progression, and encouraging patients to follow-up with their provider when necessary.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720.
2. World Health Organization. Coronavirus disease 2019 (COVID-19) situation report-75. April 4, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200404-sitrep-75-covid-19.pdf. Accessed March 24, 2022.
3. Bhimray A, Morgan RL, Shumaker AH, et al. IDSA guidelines on the treatment and management of patients with COVID-19. Updated January 18, 2022. www.idsociety.org/covid19guidelines#toc-22. Accessed January 23, 2022.
4. World Health Organization. WHO Coronavirus (COVID-19) Dashboard [online database]. 2021. https://covid19.who.int. Accessed March 5, 2022.
5. National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. www.covid19treatmentguidelines.nih.gov. Accessed March 5, 2022.
6. World Health Organization. Therapeutics and COVID-19: living guideline. 2021. www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.1. Accessed January 16, 2022.
7. CDC. COVID-19: underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare providers. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html. Accessed January 21, 2022.
8. CDC. COVID-19: Science brief: evidence used to update the list of underlying medical conditions associated with higher risk for severeCOVID-19. www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlying-evidence-table.html. Accessed January 28, 2022.
9. FDA. Fact sheet for healthcare providers: Emergency Use Authorization for Paxlovid. www.fda.gov/media/155050/download. Accessed January 15, 2022.
10. FDA. Fact sheet for healthcare providers: Emergency Use Authorization for Lagevrio (molnupiravir). www.fda.gov/media/155054/download. Accessed January 15, 2022.
11. FDA. Fact sheet for healthcare providers: Emergency Use Authorization (EUA) of Veklury (remdesivir). www.fda.gov/media/137566/download. Accessed January 15, 2022.
12. Lexicomp Online [online database]. Hudson, OH: Wolters Kluwer Health, Inc; 2019. http://online.lexi.com. Accessed January 23, 2022.
13. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386:509-520.
14. Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2022;386:305-315.
15. FDA. Fact sheet for healthcare providers: Emergency Use Authorization (EUA) of bamlanivimab and etesevimab. www.fda.gov/media/145802/download. Accessed January 22, 2022.
16. FDA. Fact sheet for healthcare providers: Emergency Use Authorization (EUA) of REGEN-COV (casirivimab and imdevimab). www.fda.gov/media/145611/download. Accessed January 21, 2022.
17. FDA. Fact sheet for healthcare providers: Emergency Use Authorization (EUA) of sotrovimab. www.fda.gov/media/149534/download. Accessed January 6, 2022.
18. FDA. Fact sheet for healthcare providers: Emergency Use Authorization for bebtelovimab. www.fda.gov/media/156152/download. Accessed March 5, 2022.
To comment on this article, contact rdavidson@uspharmacist.com.






