US Pharm. 2019;44(9)28-31.
ABSTRACT: Drug recalls occur routinely every year, and the FDA has implemented measures to ensure that these recalls are handled properly and promptly, including alerting the public, classifying the type of drug recall, and safely removing the affected product from the market. In light of the increasing number of drug recalls recently, particularly those pertaining to certain antihypertensives, it is imperative that pharmacists be acquainted with the FDA drug-recall process and have measures in place to handle recalls. Pharmacists should also be prepared to answer patient and prescriber questions and concerns when a drug recall occurs and be instrumental as well in ensuring that patient care is not interrupted or compromised.
A drug recall is the most effective means of protecting the public from a defective or potentially harmful product.1 According to the FDA, a recall is defined as an action executed by a manufacturer at any time to remove a defective or harmful drug product from the market when the drug is discovered to be in violation of laws and regulations administered by the FDA.1 Drugs may be recalled for an assortment of reasons including safety, mislabeling, contamination, and deviations in strength or potency.2 Recalls may be conducted as a voluntary action by the manufacturer or supplier; by request from the FDA; or by a legally mandated order from the FDA.2,3
The majority of drug recalls are voluntary; the manufacturer identifies an issue and recalls the affected drug. However, sometimes a drug is recalled after the FDA raises concerns.4 Regardless of the reason for the recall, the FDA’s role is to supervise a manufacturer’s strategy and assess and ensure the appropriateness of its handling of the recall.4
Approximately 80% of medications that Americans take contain some component manufactured abroad, primarily in China and India.5 While these manufacturing processes aid in keeping the costs of medications down, this has also created a supply chain that can be challenging to keep track of, which may increase the number of drug recalls.5,6 According to a recent report conducted by Kaiser Health News (KHN), thousands of drugs are recalled annually after reaching pharmacies; the recalls eventually impact patients when prescriptions are filled.7,8
Ongoing recalls have gained a noteworthy amount of attention and increased concerns among healthcare providers and the patients they treat. The KHN report states that from January 2013 to October 2018, almost 8,000 medications were recalled by pharmaceutical companies across the United States and abroad.7,8 The report also revealed that more than 2,500 facilities remained uninspected over a 5-year period, and more than 1,600 facilities, including 400 located outside the U.S., had not been inspected in 10 years.7,8
In light of the growing number of recent drug recalls involving specific lots and manufacturers of the generic versions of angiotensin II receptor blockers (ARBs) such as losartan, valsartan, and irbesartan, pharmacists are likely to receive many inquiries from concerned patients about the nature of these recalls and the possible effects on their medication.
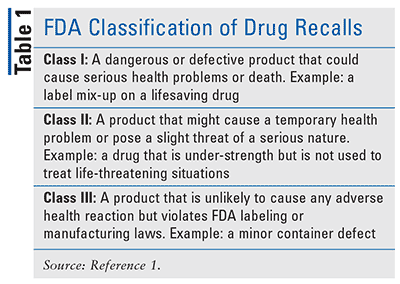
Therefore, it is imperative that pharmacists be acquainted with the FDA’s drug-recall process and be prepared to answer patient questions and address their concerns without compromising patient care. The FDA has many roles in the drug-recall process, including overseeing a manufacturer’s strategy, evaluating the appropriateness of the recall, and classifying the drug recall (Table 1).1
Selected Recalls
Valsartan: In July of 2018, the FDA began to announce a series of voluntary recalls for several lots of valsartan-containing products owing to contamination with the possible carcinogen known as N-nitrosodimethylamine (NDMA).9,10 The affected drugs were sold in the U.S. by more than a dozen pharmacies. The list of recalled drugs eventually covered more than 50% of valsartan products on the U.S. market.6,10 Other agents involved in the recent ongoing recalls included certain lots of amlodipine-valsartan combination tablets and amlodipine-valsartan-hydrochlorothiazide combination tablets that were contaminated with N-nitrosodiethylamine (NDEA).11
Unfortunately, the list of recalled products has continued to expand as more manufacturers that produce ARBs in the same class as valsartan, including products containing losartan and irbesartan, have discovered lots of drugs contaminated with NDMA or NDEA.10 Updates and press announcements regarding the ARB recalls can be found here.
Irbesartan: In late October 2018, a pharmaceutical company in India recalled 22 batches of irbesartan, which was reported to be contaminated with NDEA.6
Losartan: In early November 2018, another company recalled its losartan potassium-hydrochlorothiazide (HCTZ) product after discovering that it was contaminated with NDEA.6 On March 25, 2019, yet another pharmaceutical company issued a recall of 40 repackaged lots of losartan tablets USP 25 mg, 50 mg, and 100 mg. The voluntary recall was due to the detection of trace amounts of N-nitroso N-methyl-4-aminobutyric acid (NMBA), another possible process impurity or contaminant in an active pharmaceutical ingredient.12 The affected lots can be found on the FDA website.
Drospirenone and ethinyl estradiol tablets: Recent recalls have not been limited to ARBs. Another voluntary drug recall, to the user level, occurred in March 2019, involving four lots of drospirenone and ethinyl estradiol tablets, USP, because they might contain defective blisters with incorrect tablet arrangements and/or an empty blister pocket.13 As a result of this packaging error, where a patient does not take a tablet because it is missing or a patient takes a placebo instead of an active tablet, loss of efficacy is possible due to variation in the dosage consumed.
HCTZ: In August 2018, a voluntary recall was issued for a certain lot of HCTZ 12.5 mg owing to a labeling mix-up. The error was identified by a pharmacist who alerted the manufacturer. The lot in question actually contained spironolactone 100 mg instead of HCTZ.14 More information about this recall and affected lots can be found here.
Announcing Recalls
It is important to note that not all recalls are announced on the FDA website or via the news media, since public notification is typically released only if a recalled product has been extensively distributed or poses a serious health hazard.15 However, all recalls are listed in the FDA’s weekly enforcement report.
After the FDA classifies a recall, the agency works with the manufacturer of the recalled drug to develop a recall strategy. The strategy considers all the information learned from the FDA. It addresses the depth of the recall, public warning, and effectiveness checks.15 A recall is considered complete after all of the company’s corrective actions are reviewed by the FDA and deemed appropriate. After a recall is completed, the FDA makes sure that the product is destroyed or suitably reconditioned and investigates why the product was defective in the first place. As of July 24, 2018, the enforcement report now tracks and displays updates to a recall’s classification, reason for recall, code information, and product description if changes occur after initial publication.1
The FDA assesses the efficiency of a recall by evaluating a manufacturer’s efforts to appropriately warn customers and remove the defective product from the market.1 If a recall is determined to be ineffective, the FDA will request that the manufacturer take additional actions.1
A list of the most recent drug recalls can be found on the FDA website.
FDA Communication About Ongoing Recalls
On March 20, 2019, the FDA issued a press release indicating that to ensure patient access to losartan, the FDA will not object to certain manufacturers temporarily distributing losartan containing NMBA above the interim acceptable intake limit of 0.96 parts per million (ppm) and below 9.82 ppm until the impurity can be eliminated.16 The agency expects that many companies will be able to manufacture losartan without nitrosamine impurities and replenish the U.S. supply in approximately 6 months. Agency scientists evaluated the risk of exposure to NMBA at levels up to 9.82 ppm and determined that it presents no meaningful difference in cancer risk over a 6-month time period when compared with a lifetime of exposure to NMBA at 0.96 ppm.16 Dispensing losartan containing NMBA up to 9.82 ppm will help maintain an adequate losartan supply while companies obtain approval for manufacturing processes that produce nitrosamine-free losartan for patients.16
To address the ongoing drug recalls of ARB agents, on March 12, 2019, the FDA approved a new generic form of valsartan. For this approval, the FDA evaluated the company’s manufacturing processes and also made sure it used appropriate testing methods to demonstrate that the newly approved valsartan product does not contain NDMA or NDEA.17
In February 2019, the FDA issued a statement assuring the public that the agency is taking new steps to strengthen and modernize its process for issuing public warnings about voluntary recalls and for notification of recalls.18 This statement was created to address the FDA draft guidance issued in January 2018. The draft guidance outlined and addressed the conditions under which a company must issue a public warning about a recall, described the overall timeline for companies to issue such a warning, specified the information that should be contained within a public warning, and described situations where the FDA may take action to issue its own public warning should a company’s warning be deemed inadequate.18 The draft guidance also describes the FDA’s policy for posting recalls to the agency’s enforcement report before a final health risk determination is made.
The Role of the Pharmacist
As a front-line healthcare provider, the pharmacist can be instrumental in alerting patients and providing them with vital information about drug recalls as well as calming their fears or concerns without compromising patient care. During counseling, patients should be encouraged to contact their pharmacist for a drug replacement from an unaffected lot of their medicine, if feasible, or immediately contact their primary healthcare provider for recommendations, including a therapeutic alternative if appropriate or available.
With regard to the recent ARB drug recalls, pharmacists can relay information from the FDA about the recent recalls including the following16:
• Do not abruptly stop medication; always contact the pharmacist or primary healthcare provider.
• Continue taking current medicine until the primary healthcare provider or pharmacist provides a replacement or a different treatment option.
• Patients should be encouraged not to panic since not all valsartan-containing medications or other ARBs are affected or are being recalled.
• If a patient is taking any medication that may be recalled, he or she should compare the information on the prescription bottle with the information in the FDA recall list (company, National Drug Code, lot number) to determine if current medicine has been recalled. If not certain, contact the pharmacist.
• If the medicine is included in the recall, contact the pharmacist. The pharmacist may be able to provide a replacement from an unaffected lot or one from a different manufacturer. If not, contact the prescriber immediately to discuss other treatment options.
The FDA also wants healthcare professionals, including pharmacists, to be aware that
• The FDA has determined that the recalled valsartan products pose an unnecessary risk to patients.16 Therefore, the FDA recommends that patients use valsartan-containing medicines made by other companies or consider other available treatment options for their medical condition.
• If your pharmacy has medication samples from affected lots, quarantine the products and do not provide them to patients (see Table 2).
REFERENCES
1. FDA. FDA’s role in drug recalls. Updated July 5, 2018. www.fda.gov/Drugs/DrugSafety/DrugRecalls/ucm612550.htm. Accessed August 21, 2019.
2. Jordin R. Tips for effectively managing medication recalls. Cardinal Health. December 8, 2014. www.cardinalhealth.com/en/essential-insights/medication-recalls.html. Accessed August 21, 2019.
3. FDA. Center for Devices and Radiological Health. Recalls, corrections and removals (devices). Updated July 9, 2018. www.fda.gov/medicaldevices/deviceregulationandguidance/postmarketrequirements/recallscorrectionsandremovals/default.htm. Accessed August 21, 2019.
4. FDA. FDA 101: product recalls. www.fda.gov/ForConsumers/ConsumerUpdates/ucm049070.htm. Updated September 10, 2018. Accessed August 21, 2019.
5. American Pharmacists Association. Drug recalls put spotlight on drug supply chains. November 21, 2018. www.pharmacist.com/article/drug-recalls-put-spotlight-drug-supply-chains. Accessed August 21, 2019.
6. Smith M. Drug recalls put spotlight on drug supply chains. WebMD. November 19, 2018. www.webmd.com/a-to-z-guides/news/20181119/drug-recalls-put-spotlight-on-drug-supply-chain. Accessed March 30, 2019.
7. Lupkin S, DeMarco H. When medicine makes patients sicker. Kaiser Health News. January 4, 2019. https://khn.org/news/how-tainted-drugs-reach-market-make-patients-sicker. Accessed August 21, 2019.
8. Higgins C. Drug recalls are more widespread than previously thought. Pharmacy Today. February 19, 2019. www.pharmacist.com/article/drug-recalls-are-more-widespread-previously-thought. Accessed August 21, 2019.
9. FDA announces voluntary recall of several medicines containing valsartan following detection of an impurity. FDA website. July 13, 2018. www.fda.gov/newsevents/newsroom/pressannouncements/ucm613532.htm. Accessed August 21, 2018.
10. American College of Cardiology. Additional ARBs added to FDA recall list due to contamination. January 4, 2019. www.acc.org/latest-in-cardiology/articles/2019/01/04/16/50/additional-arbs-added-to-fda-recall-list. Accessed August 21, 2019.
11. FDA. Company announcement. Teva Pharmaceuticals USA issues voluntary nationwide recall of all amlodipine/valsartan combination tablets and amlodipine/valsartan/hydrochlorothiazide combination tablets that are within expiry. November 27, 2018. www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/teva-pharmaceuticals-usa-issues-voluntary-recall-all-amlodipinevalsartan-combination. Accessed August 21, 2019.
12. FDA. Company announcement. Legacy Pharmaceutical Packaging, LLC issues voluntary nationwide recall of losartan potassium tablets, USP, 25 mg, 50 mg, and 100 mg due to the detection of trace amounts of N-Nitroso N-Methyl 4-Amino Butyric Acid (NMBA) impurity found in the active pharmaceutical ingredient (API). March 25, 2019. www.fda.gov/Safety/Recalls/ucm633664.htm. Accessed August 21, 2019.
13. FDA. Company announcement. Apotex Corp. Issues voluntary nationwide recall of drospirenone and ethinyl estradiol tablets, USP, 28x3 blister pack/carton due to possibility of missing/incorrect tablet arrangement. March 4, 2019. www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/apotex-corp-issues-voluntary-nationwide-recall-drospirenone-and-ethinyl-estradiol-tablets-usp-28x3. Accessed August 21, 2019.
14. Medscape. August 30, 2018. Recall of hypertension meds containing wrong drug. www.medscape.com/viewarticle/901409. Accessed August 21, 2019.
15. FDA. Recalls, corrections and removals. Updated July 9, 2018. www.fda.gov/medicaldevices/deviceregulationandguidance/postmarketrequirements/recallscorrectionsandremovals/default.htm. Accessed August 21, 2019.
16. FDA. FDA updates on angiotensin II receptor blocker (ARB) recalls including valsartan, losartan and irbesartan. March 22, 2019. www.fda.gov/Drugs/DrugSafety/ucm613916.htm. Accessed August 21, 2019.
17. Wendling P. FDA fast tracks approval of generic valsartan in wake of recalls. March 12, 2019. Medscape. www.medscape.com/viewarticle/910278. Accessed August 21, 2019.
18. FDA. Statement from FDA Commissioner Scott Gottlieb, M.D., on new steps to strengthen the agency’s process for issuing public warnings and notifications of recalls. February 7, 2019. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm630906.htm. Accessed August 21, 2019.
To comment on this article, contact rdavidson@uspharmacist.com.