US Pharm. 2021;46(5):34-37.
ABSTRACT: Inflammatory bowel disease (IBD) is a gastrointestinal disease marked by cycles of active disease and remission. Patients with IBD are characterized as suffering from ulcerative colitis or Crohn’s disease. Although noncurative therapies are widely used, patient relapse and treatment failures lend to the difficulty in managing IBD. IBD treatment modalities encompass several drug classes, including anti–tumor necrosis factor (TNF) agents. These medications have become the mainstay in treating IBD. Guidelines discuss using TNF inhibitors alone or in combination with other agents in moderate-to-severe IBD. Patient education of IBD and anti-TNF agents is key to successful patient outcomes.
Inflammatory bowel disease (IBD) is a gastrointestinal (GI) disorder that is chronic, often debilitating, and characterized by disease exacerbations and remissions.1-4 These relapsing inflammatory disorders are classified as Crohn’s disease (CD) and ulcerative colitis (UC), depending on where the disease is located in the GI tract and the depth of mucosal involvement. Typical symptoms include diarrhea with or without blood, abdominal pain, weight loss, and fatigue. Treatment options include aminosalicylates, corticosteroids, immunomodulators, anti–tumor necrosis factor (TNF) agents, and integrin receptor antagonists. This article will focus on the use of anti-TNF agents, including pharmacology, dosing, administration, contraindications, and warnings, along with specific patient-education clinical pearls.
EPIDEMIOLOGY & PHARMACOLOGY OF INFLAMMATORY BOWEL DISEASE
The prevalence of IBD is increasing worldwide.1-3 Globally, there are more than 6.8 million estimated cases of IBD. Females account for 57% of cases, compared with 43% in males.3 Although the cause of IBD is not fully understood, the immune system plays a critical role, along with genetic predisposition and infectious factors.1,2 Additionally, psychological factors such as stress, anxiety, and depression appear to correlate with disease exacerbations.1,2
Overactivity of the proinflammatory pathways, dysregulation of the inflammatory cytokines, and the autoimmune cascade that follows are seen in patients with IBD. TNF-alpha, a proinflammatory cytokine, is a crucial mediator of the abnormal immune response and is increased in the mucosa and intestinal wall of patients with IBD.1,2,4,5
The inflammation noted in IBD patients leads to disruption of the intestinal mucosa and the epithelial wall barrier, causing leakiness and tissue destruction, which results in edema, ulceration, destruction of tissue, and granuloma development. This cascade signals more inflammatory cytokines to gather, leading to the histologic changes and development of the symptomatology of IBD.1,2,4-8 Anti-TNF agents block TNF-mediated activation of the proinflammatory pathways and inhibit the classic hallmarks of chronic inflammation. This results in decreased immune-mediated inflammation and may lead to mucosal healing, thus altering the natural course of IBD.4,6-8
ANTI-TNF AGENTS
Anti-TNF agents infliximab, adalimumab, certolizumab pegol, and golimumab use monoclonal antibody targeting to induce and maintain remission in patients with IBD. The efficacy of infliximab, adalimumab, and certolizumab pegol in CD has been demonstrated in various clinical trials, whereas the efficacy of infliximab, adalimumab, and golimumab has been validated in the treatment of UC.9-20 Since their arrival to the market, these agents have become a mainstay in the therapeutic treatment of IBD, have given hope to patients for controlling of their symptoms, and have improved quality of life.
Guidelines and Indications
There are four anti-TNF agents currently recommended by multiple guidelines and indicated for the treatment of moderately to severely active IBD. Infliximab and adalimumab are used in treating UC and CD.21,22 On the other hand, certolizumab pegol is only used in the treatment of CD, while golimumab is only used in the treatment of UC.23,24 A review of American Gastroenterological Association (AGA) and American College of Gastroenterology (ACG) guidelines indicates the recommendation of infliximab, adalimumab, and certolizumab pegol for induction of remission and maintenance therapy in patients with moderately severe CD.25,26 More specifically, these agents are recommended to treat CD that is resistant to treatment with corticosteroids, methotrexate, or thiopurines.26 The National Institute for Health and Care Excellence (NICE) guidelines recommend infliximab or adalimumab as therapy options for adults with severe active CD in patients who are not responsive to conventional therapy.27 Infliximab additionally plays a role in reducing the number of fistulas and maintaining fistula closure in adults with fistulizing CD.21
For the induction and maintenance of remission in moderately to severely active UC, AGA and ACG guidelines mirror the indications for these agents and recommend the use of anti-TNF agents, specifically infliximab, adalimumab, and golimumab.28,29 Indications for infliximab and golimumab explicitly highlight reducing signs and symptoms, inducing and maintaining remission and mucosal healing, and eliminating corticosteroid use in active UC patients who have experienced an inadequate response to conventional therapy.21,24 As with CD, the NICE guidelines recommend the use of these three agents to treat moderately to severely active UC in patients who fail conventional therapy.30,31
Nonresponse and continued relapses do occur in IBD patients. Up to 30% of patients show no improvement after induction therapy with anti-TNF agents, while another 30% to 40% relapse during the first year of therapy.32 Current management of these patients is mainly through empirically switching to another anti-TNF agent or biologic. A dose escalation and an increase in the frequency of administration may also be employed.32
Utilization of Anti-TNF Agents
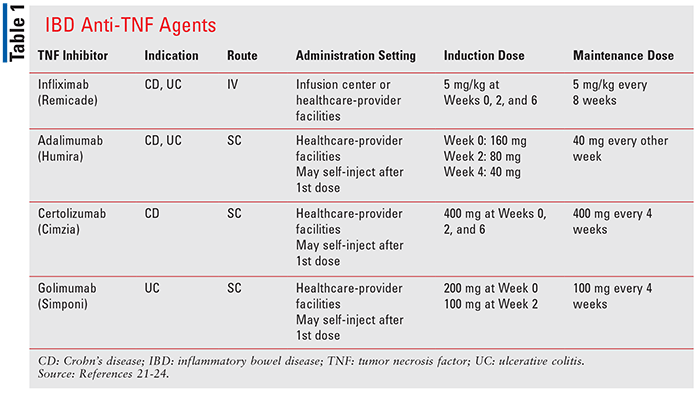
There are many other similarities and differences among these anti-TNF agents. Relative to administration, all but infliximab are given SC. Infliximab is administered as an IV infusion over at least 2 hours using an in-line filter.21 Administration of the infusion should occur only under the supervision of a healthcare provider, and medication to treat adverse reactions should be readily available. In addition, patients may be premedicated with histamine-1 receptor antagonists, histamine-2 receptor antagonists, acetaminophen, and/or corticosteroids to prevent reactions.21 Adalimumab, certolizumab pegol, and golimumab are intended to be administered under the supervision of a physician, at least for the first dose. However, unlike infliximab, if a physician determines it is appropriate and the patient has been trained on proper injection technique, self-injection at home may occur.22-24 As with route of administration, there are differences in dosing frequency. Each agent has multiweek dosing schedules for initial dosing, though the number of weeks between doses and the length of initial dosing varies. Furthermore, adalimumab, certolizumab pegol, and golimumab use a fixed-dosing approach, while infliximab’s dosing is weight-based. Details regarding dosing of these agents are shown in TABLE 1.
Because anti-TNF agents suppress the immune system, patients treated with these agents are at an increased risk of developing serious infections. In fact, all four of these agents carry a black box warning for serious infections leading to hospitalizations and death. As such, use of these agents should be discontinued if serious infection or sepsis occurs. Likewise, patients should not start treatment with anti-TNF agents if they are experiencing an active infection, including tuberculosis. Periodically during therapy, patients should be monitored and evaluated for infection. These anti-TNF agents also carry a malignancy black box warning stating lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients.21-24
The adverse reactions among these agents are comparable, with upper respiratory tract infections being one of the most common systemic effects with all four agents. Other significant adverse effects include sinusitis and pharyngitis infections, infusion-related reactions, headache and abdominal pain (infliximab); injection-site reactions, headache and rash (adalimumab); urinary tract infection (certolizumab pegol); and nasopharyngitis (golimumab).21-24 Interestingly, infliximab carries contraindications while the other three agents do not. Infliximab’s use is contraindicated in patients with moderate-to-severe heart failure for doses >5 mg/kg, as well as those incurring a previous severe hypersensitivity reaction to infliximab or any inactive ingredients or to any murine proteins.21
PATIENT EDUCATION
When initiating anti-TNF therapy, an explanation of the disease state is imperative. Patients should understand that IBD is a disease of exacerbations and remissions, and that this therapy is not curative. All patients receiving anti-TNF therapy should be provided and should read through the Medication Guide and Instructions for Use specific to the medication. Patient education should include, but is not limited to, warnings and precautions, preventative care, and if self-injecting, injection technique. Because these agents can lower the patient’s immune response to fight infections, it is extremely important to contact the prescriber if any symptoms of disease associated with tuberculosis, hepatitis B, or invasive fungal infections occur. Inform patients of the risk these agents carry for lymphoma and other malignancies. It is also paramount for patients to report signs of new or worsening health conditions, namely heart failure, liver disease, and autoimmune diseases.21-24
In addition to full blood counts, liver function tests, and renal function tests, vaccination status should be a part of the conversation regarding long-term preventative care.33 Evaluation of vaccine status should occur prior to the start of therapy. If live vaccines are deemed necessary and applicable, they should be administered 4 weeks prior to the initiation of the anti-TNF agent. Live vaccines are contraindicated in immunocompromised patients, such as those taking anti-TNF agents.34 Patients should receive an annual influenza vaccine and pneumococcal vaccinations per Advisory Committee on Immunization Practices guidelines.34 If self-injecting of agents is authorized by the prescriber, instruction of proper SC technique will be necessary and should also include rotation of injection sites, use of device, and appropriate disposal of needles and syringes.22-24
CONCLUSION
Anti-TNF agents have given hope to many who have not had control of symptoms or achievement of remission. Patients who are prescribed anti-TNF agents should be properly educated on the disease, and the anti-TNF agent prescribed, along with dosing and administration, precautions, warnings, and adverse effects. By utilizing a patient-centered approach and employing the guidelines and recommendations to direct IBD treatment with anti-TNF agents, patients can have improved outcomes and quality of life.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. Hemstreet BA. Inflammatory bowel disease. In: DiPiro JT, Yee GC, Posey L, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 11th ed. New York, NY: McGraw-Hill; 2020.
2. Tofade T, Laliberte B, Rochester-Eyeguokan C. Lower gastrointestinal disorders. In: Zeind CS, Carvalho MG, eds. Applied Therapeutics: The Clinical Use of Drugs. 11th ed. Philadelphia, PA: Wolters Kluwer Health; 2018:518-537.
3. GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17-30.
4. Magro F, Portela F. Management of inflammatory bowel disease with infliximab and other anti–tumor necrosis factor alpha therapies. BioDrugs. 2010;24(suppl 1):3-14.
5. Griffiths OR, Landon J, Coxon RE, et al. Inflammatory bowel disease and targeted oral anti-TNF alpha therapy. Protein Chem Struct Biol. 2020:119:157-98.
6. Olesen CM, Coskun M, Peyrin-Biroulet L, Nielsen OH. Mechanisms behind efficacy of tumor necrosis factor inhibitors in inflammatory bowel diseases. Pharmacol Ther. 2016;159:110-119.
7. Levin AD, Wildenberg ME, Van Den Brink GR. Mechanism of action of anti-TNF therapy in inflammatory bowel disease. J Crohns Colitis. 2016;10:989-997.
8. Rawla P, Sunkara T, Raj JP. Role of biologics and biosimilars in inflammatory bowel disease: current trends and future perspectives. J Inflamm Res. 2018;11:215-226.
9. Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541-1549.
10. Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340:1398-1405.
11. Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876-885.
12. Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52-65.
13. Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut. 2007;56:1232-1239.
14. Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC I trial. Gastroenterology. 2006;130:323-333.
15. Sandborn WJ, Feagan BG, Stoinov S, et al. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med. 2007;357:228-238.
16. Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med. 2007;357:239-250.
17. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462-2476.
18. Reinisch W, Sandborn W, Hommes D, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis. Gut. 2011;60;780-787.
19. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:85-95.
20. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:96-109.
21. Remicade [package insert]. Horsham, PA: Janssen Biotech, Inc.; 1998.
22. Humira [package insert]. North Chicago, IL: Abbott Laboratories; 2002.
23. Cimzia [package insert]. Smyrna, GA: UCB, Inc.; 2008.
24. Simponi [package insert]. Horsham, PA: Janssen Biotech, Inc.; 2009.
25. Terdiman JP, Gruss CB, Heidelbaugh JJ, et al. American Gastroenterological Association Institute guideline on the use of thiopurines, methotrexate, and anti-TNF-biologic drugs for the induction and maintenance of remission in inflammatory Crohn’s disease. Gastroenterology. 2013;145:1459-1463.
26. Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn’s disease in adults. Gastroenterology. 2018;113:481-517.
27. National Institute for Health and Care Excellence. Crohn’s disease: management. May 2019. www.nice.org.uk/guidance/ng129. Accessed February 16, 2021.
28. Feuerstein JD, Isaacs KL, Schneider Y, et al. AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. J Gastroenterol. 2020;158:1450-1461.
29. Rubin DT, Ananthakrishnan AN, Siegel C, et al. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114:384-413.
30. National Institute for Health and Care Excellence. Ulcerative colitis: management. May 2019. www.nice.org.uk/guidance/ng130. Accessed February 16, 2021.
31. National Institute for Health and Care Excellence. Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional therapy. February 2015. www.nice.org.uk/guidance/ta329. Accessed February 20, 2021.
32. Papamichael K, Gils A, Rutgeerts P, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis. 2015;21:182-197.
33. Vulliemoz M, Brand S, Juillerat P, et al. TNF-alpha blockers in inflammatory bowel diseases: practical recommendations and a user’s guide: an update. Digestion. 2020;101(suppl 1):16-26
34. CDC. Recommended Adult Immunization Schedule by Medical Condition and Other Indications, United States, 2021. www.cdc.gov/vaccines/schedules/hcp/imz/adult-conditions.html. Updated February 12, 2021. Accessed March 2, 2021.
To comment on this article, contact rdavidson@uspharmacist.com.






