US Pharm. 2020;45(10):HS-13-HS-16.
ABSTRACT: Polyadenosine phosphate–ribose polymerase (PARP) enzymes play an important role in DNA repair. The use of PARP inhibitors for gynecologic malignancies, including ovarian, primary peritoneal, and fallopian tube carcinomas, have shown efficacy in patients. Adverse events associated with PARP inhibition include hematologic toxicities, gastrointestinal toxicities, and fatigue. There are several ongoing clinical trials exploring the use of current PARP inhibitors in combination with chemotherapy, targeted agents, and immunotherapy. There are also investigational PARP inhibitors in development for gynecologic malignancies and various other solid tumors.
The knowledge of tumor genetics has dramatically shifted the treatment paradigm for patients with gynecologic malignancies through the development of polyadenosine phosphate–ribose polymerase (PARP) inhibitors. Single-strand DNA breaks leading to double-strand DNA breaks may occur in the DNA replication process. Typically, DNA repair can occur via multiple mechanisms, including a homologous recombination repair (HRR) pathway for double-strand breaks, in which PARP enzymes play a critical component. Additionally, BRCA1 and BRCA2 are considered tumor-suppressor genes and are crucial in the process of HRR to repair DNA.
In tumor cells with BRCA mutations, the HRR pathway cannot be performed; therefore, patients with germline or somatic mutations in BRCA1 or BRCA2 have increased risk of developing ovarian, breast, and other malignancies.1 To circumvent this, other DNA repair pathways are utilized by tumor cells; however, tumor cells with BRCA1 and BRCA2 mutations are sensitive to PARP inhibitors, which work by various mechanisms to increase double-strand breaks, disrupt BRCA recruitment to the site of damaged DNA, and ultimately lead to cell death.1-3
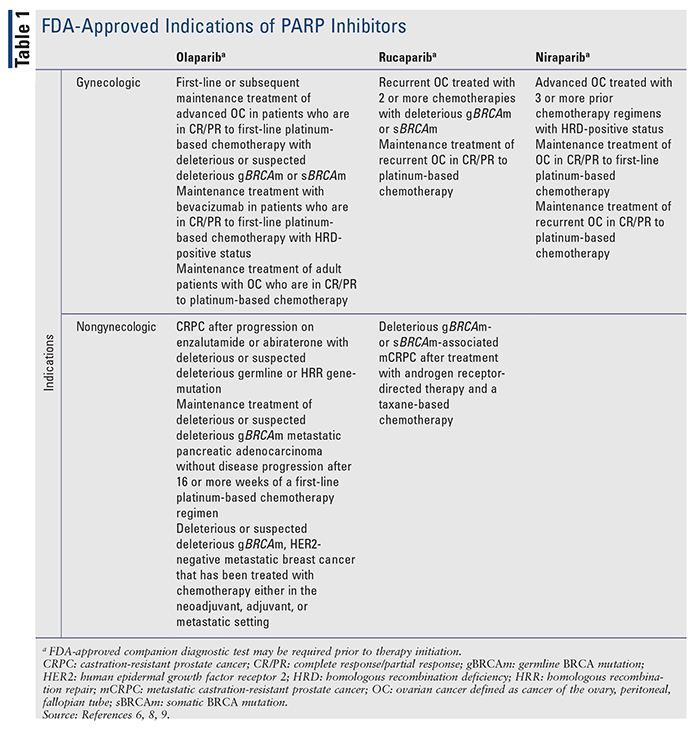
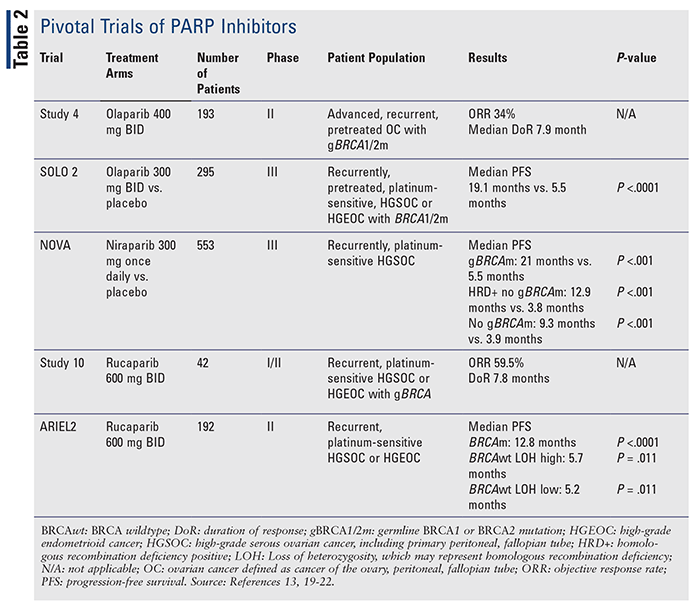
There are currently three FDA-approved PARP inhibitors for treating gynecologic malignancies: olaparib (Lynparza), rucaparib (Rubraca), and niraparib (Zejula) (TABLE 1). These approvals were based on pivotal clinical trials demonstrating efficacy (TABLE 2).3,4 The newest PARP inhibitor approved for use, talazoparib (Talzenna), is FDA approved for breast cancer.5 PARP inhibitors were initially studied in the setting of BRCA mutations in gynecologic malignancies; however, subsequent studies have demonstrated efficacy in gynecologic malignancies regardless of BRCA status.3 Furthermore, PARP inhibitors are also gaining approvals in other malignancies based on results from clinical trials, which demonstrate further potential uses for this agent class.
PARP Inhibitor Dosing
Olaparib inhibits PARP1, PARP2, and PARP3 enzymes.6 Olaparib was initially approved in capsule formulation at a dose of 400 mg twice daily with subsequent approval of the tablet form at a dose of 300 mg twice daily. The capsule formulation has since been discontinued in favor of the tablet formulation to avoid confusion.7 Niraparib is a potent inhibitor of PARP1 and PARP2.7,8 It is dosed at 300 mg once daily.8 Rucaparib inhibits PARP1, PARP2, and PARP3 and is dosed 600 mg twice daily. 3,9
Adverse Events
There are common class toxicities of PARP inhibition; however, each PARP inhibitor has a varying degree of adverse-event (AE) severity based on the interaction and binding affinities at the site of various PARP enzymes.1,3
Hematologic: Among the main AEs associated with PARP inhibition are hematologic toxicities, which occur shortly after treatment initiation and recover in a few months.3
Anemia is a common hematologic toxicity with all three PARP inhibitors; however, in phase III trials of maintenance therapy, niraparib had the highest incidence (50%), followed by olaparib (44%) and rucaparib (37%). More severe (grade 3 and 4) anemia was also more prevalent with niraparib (25%), followed by rucaparib (19%) and olaparib (19%). It is hypothesized that anemia is due to PARP2 inhibition of erythrogenesis.3
Neutropenia is another common hematologic toxicity associated with PARP inhibitors, ranging from 18% to 30%. However, severe (grade 3 and 4) neutropenias are higher with niraparib (20%), compared with rucaparib (7%) and olaparib (5%).3 It is hypothesized that the increased potency in PARP inhibition has been associated with increased myelosuppression in patients.3
Another hematologic AE of PARP inhibition is thrombocytopenia, which occurs in 61% of patients on niraparib therapy, 28% of patients on rucaparib, and 14% of patients on olaparib. Severe thrombocytopenia, grade 3 or 4, occurs at a higher frequency with niraparib (34%) compared with rucaparib (5%) and olaparib (1%).3 Baseline body weight and platelet count also predict increased thrombocytopenia in the first month of treatment with niraparib.10 Berek et al suggest a starting dose of niraparib 200 mg daily in patients weighing less than 77 kg or with platelets less than 150,000 cells/mL based on a retrospective analysis of the ENGOT-OV16/NOVA trial.10 Thrombocytopenia occurs during the first cycle for niraparib and rucaparib and plateaus after approximately three cycles.3
In general, patients should receive a baseline complete blood count (CBC) prior to initiating therapy with a PARP inhibitor.3 Since the incidence of hematologic toxicities are higher with niraparib, the FDA recommends patients undergo a weekly CBC in the first month of treatment, then monthly thereafter in the first year of treatment.3,8 It is recommended to monitor CBC monthly for rucaparib and olaparib while on therapy.6,9
Gastrointestinal: Gastrointestinal (GI) toxicities are common AEs associated with PARP inhibition. The most common GI AE is nausea, occurring in 74% to 76% of patients on PARP inhibitors. Most patients experience mild symptoms, and treatment follows chemotherapy-induced nausea and vomiting guidelines.3 Constipation, vomiting, and diarrhea are other common AEs (20%-40%) associated with PARP inhibition. Dyspepsia and dysgeusia occur more frequently with rucaparib compared with the other PARP inhibitors.3
Other AEs: Fatigue is also a common AE associated with PARP inhibition, occurring in 59% to 69% of patients. Management consists of nonpharmacologic options.3 Elevations in serum creatinine can also be seen with rucaparib and olaparib; however, it is not common and may represent an inhibition of renal transporters rather than true renal insufficiency. Niraparib is not associated with any renal dysfunction.3
Resolution of AEs
Olaparib AEs occur shortly after treatment initiation and often resolve within the first 8 weeks of therapy, making discontinuation due to AEs rare.3,7,11 Interestingly, older age did not affect the tolerability of olaparib based on an analysis of eight prospective clinical trials.12 Rucaparib AEs also occur shortly after treatment initiation and resolve after the initial cycles, with anemia occurring at the highest frequency in the first five cycles of treatment. Other hematologic and nonhematologic AEs resolve after the first three cycles.3 Toxicities with niraparib occur within the first three cycles, and nausea appears to be transient and improves over time. Thrombocytopenia is the most common AE that requires niraparib dose reduction.2,7 With niraparib, dose reductions due to AEs occur early after treatment initiation and stabilize after 3 months. Additionally, nonhematologic AEs also decrease after 3 months of treatment.3,13
Despite AEs associated with PARP inhibitor use, patient quality of life was increased compared with placebo. The quality-adjusted progression-free survival, which is a component of good quality of life, was longer with olaparib compared with placebo (13-96 months vs. 7-28 months, P <.0001).14 Additional data also demonstrated no difference in quality of life for olaparib or niraparib use as maintenance therapy for ovarian cancer.14,15
Drug Metabolism
Olaparib and rucaparib are both metabolized via the cytochrome P450 (CYP) enzymatic pathway. Olaparib induces CYP2B6 and inhibits CYP3A4.6 Rucaparib is metabolized by CYP2D6, CYP1A2, and CYP3A4.6 Concomitant medications should be reviewed prior to initiating treatment. Niraparib is metabolized in the liver by carboxylesterase-catalyzed amide hydrolysis with minimal effect on CYP450 and has no known drug interactions.3,9
Future of PARP Inhibitors
Additional PARP inhibitors under investigation for gynecologic malignancies include veliparib and talazoparib. Both investigational agents target PARP1 and PARP2. Veliparib has been evaluated in the treatment of recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients with BRCA1/2 mutations. The promising results from a phase II trial have paved the way for further investigation of veliparib in the initial and maintenance settings in a phase III study, the Gynecologic Oncology Group (GOG)-3005 trial. Talazoparib has been investigated in a phase I trial with ovarian cancer in patients with BRCA mutations with an overall response of 42%. Currently, multiple phase II and III trials are evaluating this agent in various tumor types, including gynecologic malignancies.3 Antiangiogenic agents are also being combined with PARP inhibitors. The AVANOVA trial is evaluating niraparib and bevacizumab in women with platinum-sensitive ovarian cancer.
Another promising therapy may be combining PARP inhibitors with immunotherapy. A phase II trial of olaparib in combination with durvalumab showed promising results in patients with platinum-sensitive, recurrent ovarian cancer, with the majority of patients (63%) showing a complete or partial response (n = 34).16 Patients also achieved 62% partial response or stable disease in a phase II trial looking at niraparib in combination with pembrolizumab in the platinum-resistant setting (n = 29).17
Additionally, some of the currently approved PARP inhibitors have moved into the frontline maintenance setting based on recent phase III trials, SOLO-1, PAOLA-1/ENGOT-OV25, PRIMA/ENGOT-OV26, and VELIA/GOG-3005.18
Role of the Pharmacist
The pharmacist should work collaboratively in a team setting to monitor and manage AEs due to PARP inhibitor use with the potential for dose reductions based on laboratory values or patient tolerance. Dose reduction may be necessary for olaparib due to renal dysfunction for creatinine clearance below 50 mL/min.6 Dose reductions for niraparib and rucaparib are not necessary in renal dysfunction. There are no dose-reduction recommendations for any of the three agents for hepatic dysfunction; however, in the setting of mild hepatic dysfunction, the area under the curve of olaparib was increased by 15% and may require close monitoring.6
Additionally, patient counseling for therapy adherence is of the utmost importance since all three agents are administered orally. FDA-approved PARP inhibitors for gynecologic malignancies may be taken without regard to food; however, rucaparib’s pharmacokinetic properties differ when taken with and without food due to its solubility in the small intestine, and counseling should be provided to patients for consistent adminstration.3 For patients experiencing nausea and/or vomiting, management should follow guidelines for administration of antiemetic agents.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. Lord CJ, Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science. 2017;355(6330):1152-1158.
2. Mirza MR, Pignata S, Ledermann JA. Latest clinical evidence and further development of PARP inhibitors in ovarian cancer. Ann Oncol. 2018;29(6):1366-1376.
3. LaFargue CJ, Dal Molin GZ, Sood AK, Coleman RL. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019;20(1):e15-e28.
4. Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33:244-250.
5. Talzenna (talazoparib) package insert. New York, NY: Pfizer Labs; March 2020.
6. Lynparza (olaparib) package insert. Wilmington, DE: AstraZeneca Pharmaceuticals; May 2020.
7. Franzese E, Centonze S, Diana A, et al. PARP inhibitors in ovarian cancer. Cancer Treat Rev. 2019;73:1-9.
8. Zejula (niraparib) package insert. Waltham, MA: Tesaro, Inc; April 2020.
9. Rubraca (rucaparib) package insert. Boulder, CO: Clovis Oncology, Inc; May 2020.
10. Berek JS, Matulonis UA, Peen U, et al. Safety and dose modification for patients receiving niraparib. Ann Oncol. 2018;29(8):1784-1792.
11. Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012; 366:1382-1392.
12. Dockery LE, Tew WP, Ding K, Moore KN. Tolerance and toxicity of the PARP inhibitor olaparib in older women with epithelial ovarian cancer. Gynecol Oncol. 2017;147(3):509-513.
13. Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicenter, open-label, phase 2 trial. Lancet Oncol. 2017;18(1):75-87.
14. Friedlander M, Gebski V, Gibbs E, et al. Health-related quality of life and patient-centred outcomes with olaparib maintenance after chemotherapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT Ov-21): a placebo-controlled, phase 3 randomised trial. Lancet Oncol. 2018;19(8):1126-1134.
15. Oza AM, Matulonis UA, Malander S, et al. Quality of life in patients with recurrent ovarian cancer treated with niraparib versus placebo (ENGOT-OV16/NOVA): results from a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2018;19(8):1117-1125.
16. Drew Y, de jonge M, Hong S-H, et al. An open-label, phase II basket study of olaparib and durvalumab (MEDIOLA): results in germline BRCA-mutated (gBRCAm), platinum-sensitive relapsed (PSR) ovarian cancer (OC). Gynecol Oncol. 2018;149(1):246-247.
17. Konstantinopoulos PA, Munster P, Forero-Torez, et al. TOPACIO: preliminary activity and safety (pts) in patients with platinum-resistant ovarian cancer (PROC) in a phase 1/2 study of niraparib in combination with pembrolizumab. J Clin Oncol. 2018;36(15 suppl):106-106.
18. Mirza MR, Coleman RL, González-Martín A, et al. The forefront of ovarian cancer therapy: update on PARP inhibitors. Ann Oncol. 2020;S0923-7534(20)39891-4.
19. Domchek SM, Aghajanian C, Shapira-Frommer R, et al. Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy. Gynecol Oncol. 2016;140(2):199-203.
20. Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomized, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(9):1274-1284.
21. Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrently ovarian cancer. N Engl J Med. 2016;375:2154-2164.
22. Kristeleit R, Shapiro GI, Burris HA, et al. A phase I-II study of the oral PARP inhibitor rucaparib in patients with germline BRCA1/2-mutated ovarian carcinoma or other solid tumors. Clin Cancer Res. 2017;23(15):4095-4106.
To comment on this article, contact rdavidson@uspharmacist.com.






