Acute coronary syndromes (ACS) include the spectrum of clinical conditions ranging from unstable angina to non-Q-wave myocardial infarction (MI) and Q-wave MI. Each year, more than 500,000 patients present with a new MI.1 A large number of these patients will experience recurrent MI (23%), heart failure (30%), or sudden death (6%) within six years of the initial event.1,2 Additional complications after acute MI include cardiac arrhythmias, stroke, diminished quality of life, and increased economic burden.2
Long-term pharmacologic management
after an acute MI involves four pharmacologic drug categories: antiplatelet
therapy, angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, and
statins. Despite substantial scientific evidence, guideline recommendations,
and findings from national registries, secondary preventive strategies
continue to be underutilized. The national registry CRUSADE (Can Rapid risk
stratification of Unstable angina patients Suppress ADverse outcomes with
Early implementation) of the
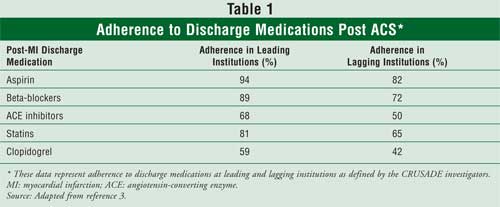
The purpose of this article is to provide pharmacists with a review of ACC/AHA guidelines for the pharmacologic management of patients who have had ACS.
A Review of Pharmacotherapy
Antiplatelet Therapy:
At hospital discharge, patients who have had ACS should continue antiplatelet
therapy with aspirin and clopidogrel for secondary prevention of
cardiovascular events.4,5
Aspirin irreversibly inhibits the cyclooxygenase enzyme, resulting in decreased production of thromboxane A2 and, thereby, decreased platelet aggregation.6 However, the antiplatelet effects of aspirin are weak, since it does not inhibit platelet activation caused by thromboxane A2-independent pathways, such as adenosine diphosphate or thrombin. Nonetheless, the ACC/AHA guidelines for secondary prevention of coronary disease recommend aspirin 75 to 162 mg daily, with treatment continuing indefinitely.7 A larger dose of 325 mg is recommended in patients who have received percutaneous coronary intervention (PCI) with stent placement, due to greater thrombotic risk.4,7
Compared with aspirin, the antiplatelet effects of clopidogrel are attributable to a different but complimentary pathway of platelet activation. Adenosine diphosphate activates platelets through the P2Y receptor; clopidogrel irreversibly binds to this receptor, thereby decreasing platelet activation. The Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial compared the effects of aspirin and clopidogrel in more than 19,000 patients with recent MI, ischemic stroke, or peripheral artery disease.8 The primary end point was the prevention of MI, ischemic stroke, or vascular death. Patients receiving clopidogrel demonstrated a relative risk reduction of 8.7% in the primary end point, suggesting that clopidogrel is at least as effective as aspirin for secondary prevention of cardiovascular events. Therefore, patients who are intolerant to aspirin due to a contraindication (i.e., gastrointestinal bleed) or hypersensitivity should receive clopidogrel 75 mg daily as an alternative.4
Several studies support the combination of aspirin plus clopidogrel for the additive antiplatelet effect. For instance, the Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial compared the effects of aspirin plus clopidogrel to aspirin alone on the composite end point of cardiovascular death, MI, and stroke.9 Patients in the combination group had significantly fewer cardiovascular events than did those in the aspirin group (9.3% vs.11.4%; P <0.001); combination therapy was associated with a 20% relative risk reduction. Although aspirin plus clopidogrel was associated with an increased risk of major and minor bleeding, there was no difference in life-threatening bleeding. A subgroup analysis, PCI-CURE, found that patients who underwent PCI received significantly greater benefit with clopidogrel plus aspirin compared with aspirin alone.10 Another trial, Clopidogrel for the Reduction of Events During Observation (CREDO), studied patients undergoing elective PCI. Patients received clopidogrel plus aspirin or aspirin alone for up to 12 months. A relative risk reduction of 26.9% was observed among those receiving combination therapy.11 Thus, it is important for practitioners to evaluate the risks and benefits of combination therapy. The ACC/AHA guidelines recommend clopidogrel 75 mg daily for up to 12 months in combination with aspirin to prevent secondary cardiovascular events in patients who have had ACS.7
ACE Inhibitors: The activation of the renin-angiotensin-aldosterone system (RAAS) has clinical implications for patients with ACS, increasing their risk of recurrent cardiovascular events.12 Blockade of this system has been shown to extend survival and curtail adverse outcomes in patients with left ventricular dysfunction and systolic heart failure, a population often at risk for cardiovascular complications, including ACS.13,14
ACE inhibitors possess direct cardiovascular protective effects by increasing vasodilator bradykinin concentration, reducing plasma aldosterone concentration via blockade of the RAAS, and reducing the vasoconstrictor angiotensin II concentration. These pharmacologic effects may lead to antiatherosclerotic effects, improved endothelial function, stabilization of plaques, fibrinolysis, and reduced neointimal formation.15
Clinical trials have established the beneficial role of ACE inhibitors in the secondary management of ACS when added early to conventional treatment such as aspirin, beta-blockers, and statins. Trials involving ramipril, trandolapril, captopril, and lisinopril therapy have shown improved survival and reduced morbidity among patients who have had an MI with left ventricular dysfunction. The Heart Outcomes Prevention Evaluation (HOPE) study enrolled 9,297 high-risk patients with evidence of vascular disease or diabetes plus one other cardiovascular risk factor who were not known to have heart failure or low ejection fraction. Ramipril significantly reduced the rates of death, MI, stroke, coronary revascularization, cardiac death, heart failure, diabetes, and complications related to diabetes.16 The European Trial on Reduction of Cardiac Events with Perindopril in Stable Coronary Artery Disease (EUROPA) study expanded on the results of the HOPE study by assessing the benefit of the ACE inhibitor perindopril in low-risk patients with stable coronary heart disease, hypertension, diabetes, and angina but without heart failure. Perindopril was shown to reduce cardiovascular death, MI or cardiac arrest, and nonfatal MI. 15
However, the benefits observed in the HOPE and EUROPA trials contrasted with findings from the Prevention of Events with Angiotensin-Converting Enzyme Inhibition (PEACE) trial, which showed no evidence of improved outcomes in patients with preserved left ventricular function who received standard therapy plus trandolapril.17 Consequently, the AHA/ACC guidelines for secondary prevention of coronary artery disease recommend the use of ACE inhibitors in patients with left ventricular ejection fraction less than 40%, and well as in those with diabetes, hypertension, or chronic kidney disease. ACE inhibitors should be initiated within 24 hours of admission and continued indefinitely in these patients unless systolic blood pressure is less than 100 mmHg or bilateral renal artery stenosis is present. Controversy remains regarding initiating ACE inhibitors in patients with stable coronary disease and preserved left ventricular function following revascularization; in these patients, ACE inhibitors are an option.7 For patients who are unable to tolerate ACE inhibitors, and who have heart failure or left ventricular ejection fraction less than 40%, angiotensin II receptor blockers (ARBs) are an alternative treatment modality. Additonally, for patients with systolic dysfunction heart failure, the combination of an ARB and an ACE inhibitor can be utilized.7
Beta-Blockers: Beta-blockers have been shown to reduce recurrent MI, cardiovascular death along with atrial or ventricular arrhythmia, and sudden cardiac death. 18,19 These benefits are thought to be achieved through decreases in heart rate, blood pressure, and myocardial contractility, improved diastolic filling, and, thereby, a decrease in myocardial workload.
The majority of clinical trial data supporting the use of beta-blockers began to accumulate in the early 1980s and comprised trials using propranolol, timolol, and metoprolol.18,20-22 In the Norwegian Multicenter Study (NMS), patients who had experienced an MI were randomized to timolol 10 mg twice daily (n = 945) or placebo (n = 939) seven to 28 days after the infarction. Patients were followed for an average of 17 months. Timolol significantly reduced mortality and recurrent MI, compared with placebo. The results showed a 39% reduction in total mortality (P <0.05) and a 28% reduction in recurrent MI.20 Additionally, the mortality benefit was observed through six years of treatment.22
Similarly, in the Beta-Blocker Heart Attack Trial (BHAT), there was an overall reduction of mortality, cardiovascular mortality, and nonfatal reinfarction rates compared with placebo. In BHAT, patients who had experienced MI were randomized to treatment with propranolol (n = 1,916) or placebo (n = 1,912) five to 21 days after the infarction. Throughout the trial, the maintenance dosage of propranolol was either 180 or 240 mg/day; however, this was dependent on individual patient serum samples. Patients were followed for an average of 24 months. Results demonstrated a 26% reduction in total mortality (P <0.05), a 27% reduction in cardiovascular mortality (P <0.05), and a 23% reduction in the rate of nonfatal reinfarction.21
The abundance of clinical trials involving beta-blockers was later distilled into a meta-analysis. A systematic review of trials capturing 54,234 patients receiving beta-blockers or control following MI demonstrated a 23% reduction in the long-term odds of death. For every 42 patients treated over a two-year period, one death was prevented. 23
Despite scientific evidence supporting the use of beta-blockers, literature still shows their underutilization.3 This underutilization is estimated to cost 3,000 to 5,000 lives in the first year following an MI due to lack of beta-blocker use.24 Underutilization is erroneously driven by several factors: fear of hypotension and bradycardia, age (persons older than 65 are less likely to receive a beta-blocker), and certain comorbid conditions. Patients with chronic obstructive pulmonary disease/asthma, diabetes, or heart failure are less likely to receive a beta-blocker due to fears of worsening their condition.18
Beta-blockers approved by the FDA for the secondary prevention of MI include atenolol, metoprolol tartrate, timolol, and propranolol. Doses of beta-blockers should reflect those used in clinical trials. Up-titration to goal doses should occur slowly and no sooner than every two weeks. The target dosage of atenolol is 100 mg/day; dosages of metoprolol tartrate, timolol, and propranolol are 200 mg/day, 20 mg/day, and 240 mg/day, respectively. In addition, carvedilol is FDA approved for patients with left ventricular dysfunction following an MI, with a target dosage of 50 mg/day.18 The ACC/AHA guidelines recommend indefinite use of beta-blockers to prevent secondary cardiovascular events in patients who have had ACS.7
Statins: Statins share two major mechanisms of action by which serum cholesterol is lowered. Statins reduce hepatic cholesterol synthesis through inhibition of the HMG-CoA reductase enzyme--thereby blocking mevalonate, which is necessary for cholesterol synthesis--and by increasing hepatic low-density lipoprotein (LDL) cholesterol receptor activity, which increases the binding sites for hepatic LDL cholesterol uptake. The value of statin therapy has been under close scientific scrutiny for more than 20 years due to the preponderance of recent evidence suggesting clear, solid evidence of benefit.25 Epidemiologic evidence suggests a linear relationship between LDL cholesterol reduction and cardiovascular risk; every 40 mg/dL reduction in LDL cholesterol translates into a 20% or greater reduction in coronary and vascular events. 26 Moderate-dose statin therapy has been associated with a 26% reduction in the odds of a clinical cardiovascular event, with an overall 3.82% reduction in absolute risk. For every 27 people with established cardiovascular disease who are treated with moderate-dose statin therapy as a secondary prevention strategy, one death, nonfatal MI, or stroke can be avoided.27 For every 35 people treated using primary prevention strategies, one death, non-fatal MI, or stroke can be avoided.28
Patients with established coronary artery disease or coronary artery disease risk equivalents require a minimal LDL cholesterol reduction to levels less than 100 mg/dL, which is possible when both pharmacotherapy and lifestyle interventions are followed.29 Individuals with coronary artery disease who also have diabetes or metabolic syndrome or are active smokers are considered to be at the highest risk for a secondary event. These individuals typically have LDL cholesterol goals lower than 70 mg/dL.30 Intensive statin therapy (80-mg doses of atorvastatin or simvastatin) is an option to achieve more aggressive LDL cholesterol goals. Higher doses provide small incremental benefits above and beyond moderate-dose statin therapy, with an additional 15% reduction in the odds of cardiovascular death and MI and an additional 18% reduction in the odds of stroke.31 Despite greater therapeutic efficacy, intensive statin therapy may not be an option for all high-risk patients due to the increase of statin-related adverse events. While these adverse events are reversible and manageable, they may affect compliance; thus, the use of complimentary mechanisms for LDL cholesterol reduction will be necessary to achieve lower LDL cholesterol goals.
The most widely used combination is that of a statin plus ezetimibe, a cholesterol absorption inhibitor. Ezetimibe reduces the impact of dietary cholesterol on total serum cholesterol by inhibiting the absorption of dietary cholesterol at the level of the intestinal brush border. In tandem, this combination provides three mechanisms that drive reductions in cholesterol: (1) reduction of hepatic cholesterol synthesis (statins); (2) increased hepatic LDL cholesterol uptake (statins); and (3) reduction of dietary cholesterol absorption at the level of the intestinal brush border (ezetimibe). A recent posthoc analysis comparing the combination of ezetimibe and simvastatin (10 mg/20 mg) against 10 to 20 mg of atorvastatin or simvastatin alone studied the proportion of patients achieving LDL cholesterol targets of less than 100 mg/dL and less than 70 mg/dL at six weeks in 1,498 high-risk patients.32-34 Eighty-three percent of patients receiving combination therapy achieved an LDL cholesterol goal of less than 100 mg/dL, whereas only 45% of patients who received atorvastatin or simvastatin achieved this minimally acceptable goal. Achieving LDL cholesterol goals of less than 70 mg/dL is considerably more difficult with either statin monotherapy or combination therapy. Thirty percent to 34% of patients receiving combination therapy achieved an LDL cholesterol goal of less than 70 mg/dL, compared with only 5% of patients receiving monotherapy. It should be expected that more patients will achieve aggressive LDL cholesterol goals with combination therapy rather than with monotherapy; however, the absolute proportion of patients achieving these goals suggests that pharmacotherapy alone is not sufficient for the majority of patients. It is also important to note that hard outcome data regarding the avoidance of cardiovascular events have not yet been documented with combination therapy comprising statins and cholesterol absorption inhibitors.
Adjunctive Measures: Omega-3 fatty acid therapy has been shown to stabilize atherosclerotic plaques, decrease myocardial ischemia, and provide membrane-stabilizing effects that reduce cardiac arrhythmias.35 The Gruppo Italiano per lo Studio della sopravivenza nell'Infarcto miocardio (GISSI) trial enrolled 11,324 patients with recent MI. Findings showed that omega-3 fatty acids reduced the risk of death, nonfatal acute MI, or nonfatal stroke. Therefore, the ACC/AHA guidelines recommend the use of 1 g/day of omega-3 fatty acids for the secondary prevention of cardiovascular events.35,36
Another adjunct measure is nitroglycerin sublingual therapy, which has been utilized for the relief of chest pain; however, no data have shown reduction in cardiovascular events. Nonetheless, the ACC/AHA guidelines recommend that all patients who have had an MI be prescribed sublingual therapy to relieve symptoms of angina when necessary.4
Conclusion
In summary, the risk of coronary heart disease events such as recurrent MI,
stroke, and death are greater for patients with a history of MI and
established coronary disease than for patients without known coronary disease;
thus, all patients who have had ACS require the administration of
antiplatelet, anti-ischemic, and lipid-lowering therapy unless
contraindications are present. In addition, omega-3 fatty acids and
nitroglycerin should be considered as adjunctive modalities. Pharmacists
should take an active role in achieving optimal patient safety and outcomes by
ensuring that these therapies are implemented.
References
1. Thom T, Haase N,
Rosamond W, et al. Heart disease and stroke statistics--2006 update: a report
from the American Heart Association Statistics Committee and Stroke Statistics
Subcommittee. Circulation. 2006;113:e85-e151.
2. Amin A. Improving the management of patients after myocardial infarction,
from admission to discharge. Clin Ther. 2006;28:1509-1539.
3. Ohman EM, Roe MT, Smith SC Jr, et al. Care of non-ST-segment elevation
patients: insights from the CRUSADE national quality improvement initiative.
Am Heart J. 2004;148(5 suppl):S34-S39.
4. Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA 2002 guideline update
for the management of patients with unstable angina and non-ST-segment
elevation myocardial infarction--summary article: a report of the American
College of Cardiology/American Heart Association Task Force on Practice
Guidelines (Committee on the Management of Patients With Unstable Angina).
J Am Coll Cardiol. 2002;40:1366-1374.
5. Volturo GA, Mazzola JL, Przylenk K. The role of antiplatelet therapy in the
management of acute coronary syndromes. Expert Opin Drug Saf.
2005;4:541-556.
6. Patrono C. Aspirin as an antiplatelet drug. N Engl J Med.
1994;330:1287-1294.
7. AHA; ACC; National Heart Lung, and Blood Institute; Smith SC Jr, Allen J,
Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with
coronary and other atherosclerotic vascular disease: 2006 update endorsed by
the National Heart, Lung, and Blood Institute. J Am Coll Cardiol.
2006;47:2130-2139.
8. A randomised, blinded, trial of clopidogrel versus aspirin in patients at
risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet.
1996;348:1329-1339.
9. Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to
aspirin in patients with acute coronary syndromes without ST-segment
elevation. N Engl J Med. 2001;345:494-502.
10. Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with
clopidogrel and aspirin followed by long-term therapy in patients undergoing
percutaneous coronary intervention: the PCI-CURE study. Lancet.
2001;358:527-533.
11. Steinhubl SR, Berger PB, Mann JT 3rd, et al. Early and sustained dual oral
antiplatelet therapy following percutaneous coronary intervention: a
randomized controlled trial. JAMA. 2002;288:2411-2420.
12. Lonn EM, Yusuf S, Jha P, et al. Emerging role of angiotensin-converting
enzyme inhibitors in cardiac and vascular protection. Circulation.
1994;90:2056-2069.
13. Effect of enalapril on survival in patients with reduced left ventricular
ejection fractions and congestive heart failure. The SOLVD Investigators. N
Engl J Med. 1991;325:293-302.
14. Effect of enalapril on mortality and the development of heart failure in
asymptomatic patients with reduced left ventricular ejection fractions. The
SOLVD Investigators. N Engl J Med. 1992;327:685-691.
15. Fox KM. Efficacy of perindopril in reduction of cardiovascular events
among patients with stable coronary artery disease: randomised, double-blind,
placebo-controlled, multicentre trial (the EUROPA study). Lancet.
2003;362:782-788.
16. Yusuf S, Sleight P, Pogue J, et al. Effects of an
angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in
high-risk patients. The Heart Outcomes Prevention Evaluation Study
Investigators. N Engl J Med. 2000;342:145-153.
17. Braunwald E, Domanski MJ, Fowler SE, et al; The Peace Trial Investigators.
Angiotensin-converting-enzyme inhibition in stable coronary artery disease.
N Engl J Med. 2004;351:2058-2068.
18. Everly MJ, Heaton PC, Cluxton RJ Jr. Beta-blocker underuse in secondary
prevention of myocardial infarction. Annal Pharmacother.
2004;38:286-293.
19. Stringer KA, Lopez LM. Uncomplicated myocardial infarction. In: Dipiro JT,
ed. Pharmacotherapy: A Pathophysiologic Approach. New York, NY:
McGraw-Hill; 2002.
20. Timolol-induced reduction in mortality and reinfarction in patients
surviving acute myocardial infarction. N Engl J Med. 1981;304:801-807.
21. A randomized trial of propranolol in patients with acute myocardial
infarction. I. Mortality results. JAMA. 1982;247:1707-1714.
22. Pedersen TR. Six-year follow-up of the Norwegian Multicenter Study on
Timolol after Acute Myocardial Infarction. N Engl J Med.
1985;313:1055-1058.
23. Freemantle N, Cleland J, Young P, et al. Beta Blockade after myocardial
infarction: systematic review and meta regression analysis. BMJ.
1999;318:1730-1737.
24. Bradford WD, Chen J, Krumholz HM. Under-utilisation of beta-blockers after
acute myocardial infarction. Pharmacoeconomic implications.
Pharmacoeconomics. 1999;15:257-268.
25. Steinberg D. Thematic review series: the pathogenesis of atherosclerosis.
An interpretive history of the cholesterol controversy, part V: the discovery
of the statins and the end of the controversy. J Lipid Res.
2006;47:1339-1351.
26. Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of
cholesterol-lowering treatment: prospective meta-analysis of data from 90,056
participants in 14 randomised trials of statins. Lancet.
2005;366:1267-1278.
27. Silva MA, Swanson AC, Gandhi PJ, Tataronis GR. Statin-related adverse
events: a meta-analysis. Clin Ther. 2006;28:26-35.
28. Statin outcome trials update. Bandolier, 2006. Available at
www.jr2.ox.ac.uk/bandolier/booth/cardiac/statout.html. Accessed December 3,
2006.
29. Executive summary of the Third Report of the National Cholesterol
Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment
of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA.
2001;285:2486-2497.
30. Grundy SM, Cleeman JI, Merz CN, et al; National Heart, Lung, and Blood
Institute; American College of Cardiology Foundation; American Heart
Association. Implications of recent clinical trials for the National
Cholesterol Education Program Adult Treatment Panel III guidelines.
Circulation. 2004;110:227-239.
31. Silva MA, Matthews ML, Jarvis C, et al. Meta-analysis of drug induced
adverse events associated with intensive dose statin therapy. Clin Ther
. 2007;in press.
32. McKenney J, Ballantyne CM, Feldman TA, et al. LDL-C goal attainment with
ezetimibe plus simvastatin coadministration vs atorvastatin or simvastatin
monotherapy in patients at high risk of CHD. MedGenMed. 2005;7:3.
33. Ballantyne CM, Blazing MA, King TR, et al. Efficacy and safety of
ezetimibe co-administered with simvastatin compared with atorvastatin in
adults with hypercholesterolemia. Am J Cardiol. 2004;93:1487-1494.
34. Feldman T, Koren M, Insull W Jr, et al. Treatment of high-risk patients
with ezetimibe plus simvastatin co-administration versus simvastatin alone to
attain National Cholesterol Education Program Adult Treatment Panel III
low-density lipoprotein cholesterol goals. Am J Cardiol.
2004;93:1481-1486.
35. Jacobson TA. Secondary prevention of coronary artery disease with omega-3
fatty acids. Am J Cardiol. 2006;98:61i-70i.
36. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E
after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo
Italiano per lo Studio della Sopravivenza nell'Infarto miocardico. Lancet
. 1999;354:447-455.
To comment on this article, contact editor@uspharmacist.com.





