US Pharm. 2021;46(9):21-25.
ABSTRACT: Premenstrual disorders are common among premenopausal women and can have a major impact on their daily lives. Premenstrual syndrome (PMS) and premenstrual dysphoric disorder (PMDD) can cause physical symptoms and psychological symptoms similar to those of depression, with PMDD being the most severe form of PMS. Treatment approaches range from lifestyle changes and self-care to behavioral therapies and prescription medications. Many patients express a preference for OTC supplements as a more natural approach to treating their symptoms. Selective serotonin reuptake inhibitors are the gold standard for pharmacologic treatment of PMS and PMDD. Pharmacist knowledge of these various therapies is invaluable for informing patients’ treatment selection, medication adherence, and therapeutic progress.
Premenstrual disorders are common among premenopausal women and can have a major impact on home, work, or school life and interpersonal relationships. Both premenstrual syndrome (PMS) and premenstrual dysphoric disorder (PMDD) involve physical and psychological symptoms that occur during the luteal phase of ovulation, resolve shortly after menstruation begins, and affect normal daily functioning.1-4 The American College of Obstetrics and Gynecology defines PMS as a disorder in which a woman experiences at least one physical symptom and one emotional symptom that impact social, academic, or work performance, whereas PMDD is a severe form of PMS involving at least five emotional and physical symptoms that are significant enough to cause major functional impairment or disruptions to daily life.3-7 For a diagnosis of PMDD, one of the key emotional symptoms must be mood swings, anger, irritability, depression, or anxiety.4,7,8
Incidence and Prevalence
Approximately 80% of women report at least one physical or emotional symptom during the luteal phase of the menstrual cycle, and most of them do not experience a significant impact on daily functioning. It is estimated that 20% to 25% experience moderate-to-severe symptoms that affect some daily functioning (i.e., PMS), and 1.2% to 8% have received a clinical diagnosis of PMDD. PMDD patients report severe, disabling symptoms that affect their daily life.2,3,7,9 PMS prevalence is not associated with age, level of education, or employment status.3,4,7,10
Risk Factors
Several risk factors for the development of PMS have been identified. These risk factors include a family history of other affective disorders, including major depression and postpartum depression; physical, sexual, or emotional trauma; weight gain; Caucasian race; nicotine use; and having experienced a stressful event in the past year.2-4,7
Etiology
The etiology of PMS and PMDD is unclear, but several theories have been proposed.3-5,7 Cyclical fluctuations in levels of estrogen and progesterone may trigger the symptomology of PMS. In addition, decreased sensitivity to the circulating hormones may play a role. Serotonin levels drop during the mid-to-late luteal phase, and the lower density of serotonin transporters results in abnormal serotonergic transmission. Luteal-phase changes in the brain neurocircuitry that affect emotional and cognitive functioning may be involved as well. Lastly, a genetic predisposition has been noted.3-5,7
Evaluation and Diagnosis
Patient evaluation should include a thorough history of present illness. Other factors in the patient’s medical history also must be considered with regard to the differential diagnosis. Ruling out premenstrual exacerbation of any underlying psychiatric disorders is paramount; therefore, the patient should be screened for mood disorders such as major depressive disorder, generalized anxiety disorder, dysthymic disorder, and seasonal affective disorder. In addition, the patient should be queried about various medical comorbidities that can mimic premenstrual symptoms or may worsen during the luteal phase of the menstrual cycle, including migraines, anemia, thyroid disease (hyperthyroidism or hypothyroidism), endometriosis, irritable bowel syndrome, chronic fatigue syndrome, rheumatologic disorders, and chronic pain.1-3,6,7 A diagnosis of PMS can be made if the patient has physical symptoms that cause functional impairment even in the absence of emotional symptoms.4 Once a provisional or preliminary diagnosis of PMS has been made, the patient should prospectively chart her daily symptoms over two menstrual cycles. A definitive diagnosis is based on the patient’s history and completed daily symptom diary.3-5,7
Symptoms
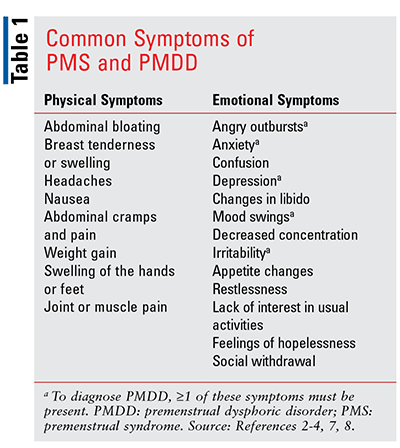
Common physical symptoms of PMS and PMDD include abdominal bloating, breast tenderness or swelling, headaches, nausea, abdominal cramps and pain, weight gain, swelling of the hands or feet, and joint or muscle pain. Common emotional symptoms of these disorders include angry outbursts, anxiety, confusion, depression, libidinal changes, mood swings, decreased concentration, irritability, appetite changes, confusion, restlessness, lack of interest in usual activities, feelings of hopelessness, and social withdrawal.2,3 See TABLE 1 for a listing of common symptoms. The symptoms usually occur in the week prior to menstruation, start lessening when menstruation begins, and are minimal or nonexistent following menstruation. The peak symptoms normally occur from 2 days prior to, through the first day of, menstruation.3-5,7
Management
Treatment plans for PMS and PMDD encompass nonpharmacologic and pharmacologic approaches used either together or separately. Self-treatment may include consistent exercise, vitamin and mineral supplementation, and relaxation therapy. Pharmacologic treatment is suggested when lifestyle changes and other nonpharmacologic therapies no longer provide adequate symptom relief or if the patient prefers to initiate therapy with a prescription medication instead of nonpharmacologic measures.11 Pharmacologic options include serotonergic antidepressants, anxiolytics, and hormonal therapies.6,11 Treatment should be based on symptom severity and duration, menstrual-cycle regularity, whether contraception is also desired, and whether the patient is willing to take medication.4,12
Nonpharmacologic Treatment
Self-treatment of PMS and PMDD ranges from lifestyle modifications and cognitive-behavioral therapy (CBT) to OTC supplements. Although the evidence for alternative therapies and self-treatment options for PMS and PMDD is weak, the use of alternative therapies as concomitant treatment is widely accepted.7,8,12 The use of CBT has been shown to enhance the ability of PMS and PMDD patients to modify irrational thoughts and develop better coping strategies.8,12 Dietary changes, such as consuming complex carbohydrates rather than simple carbohydrates, reducing salt intake, and eliminating caffeine, have theoretical effects on reducing symptoms such as bloating and mood swings; however, evidence is lacking and more research is needed.7,12 Whereas the Royal College of Obstetricians and Gynaecologists suggests that exercise, CBT, and vitamin B6 be considered first-line treatment for PMS, other experts have concluded that the body of evidence is too limited to recommend these options for first-line use.12,13
Although many patients express a preference for OTC supplements as a more natural approach to treating PMS and PMDD symptoms, the evidence for these products is limited and inconclusive. Calcium 600 mg twice daily carries the strongest evidence for OTC treatment of mood symptoms associated with PMS and PMDD.8,12 Other supplements that may provide some benefit for symptoms include vitamin B6, omega-3 fatty acids, evening primrose oil, ginkgo biloba, and chasteberry; however, the consensus is that more evidence is needed for all of these treatment alternatives.7,8,12
Additional complementary therapies that may be considered for PMS and PMDD treatment include light therapy, acupuncture or acupressure, massage, yoga, and chiropractic care; however, all of these treatment options require further evidence to determine their effectiveness for treating symptoms of PMS and PMDD.7,14
Pharmacologic Treatment
Similar to their use for major depression, selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and benzodiazepines (BZDs) are used to treat PMS and PMDD. Other pharmacologic classes include oral contraceptives (OCs) and gonadotropin-releasing hormone (GnRH) agonists.5,6,11 SSRIs, which may be dosed either intermittently or continuously, are considered the primary treatment option for PMDD and severe PMS.6,8,15 Intermittent dosing is an effective strategy because SSRIs have a short onset of action of just hours or days in PMDD treatment; this is unlike the response seen in the treatment of major depression, in which full onset of action can take several weeks.8,16 In intermittent dosing for PMDD, the medication is taken in the luteal phase (i.e., from ovulation until the beginning of menses) only.12 Intermittent dosing appears to be more efficacious for symptoms of irritability and mood swings and less beneficial for depressed mood and somatic symptoms. Therefore, continuous dosing may be a better option when depressed mood and somatic symptoms are present.8
Currently, the FDA has approved three SSRIs for treatment of PMDD: fluoxetine, sertraline, and paroxetine (TABLE 2).16 The off-label SSRI agents citalopram and escitalopram are also effective, and the SNRI venlafaxine is an alternative primary option (TABLE 2).5,6,12 In general, side effects from SSRIs and SNRIs are common upon initiation of therapy; these include nausea, insomnia, headache, fatigue, diarrhea, dizziness, and decreased libido.3,12 With the exception of decreased libido, which may be a long-term effect and can adversely impact medication adherence, most side effects subside in a matter of days regardless of dosing strategy. The burden of side effects can be further reduced with use of luteal-phase dosing.6,12
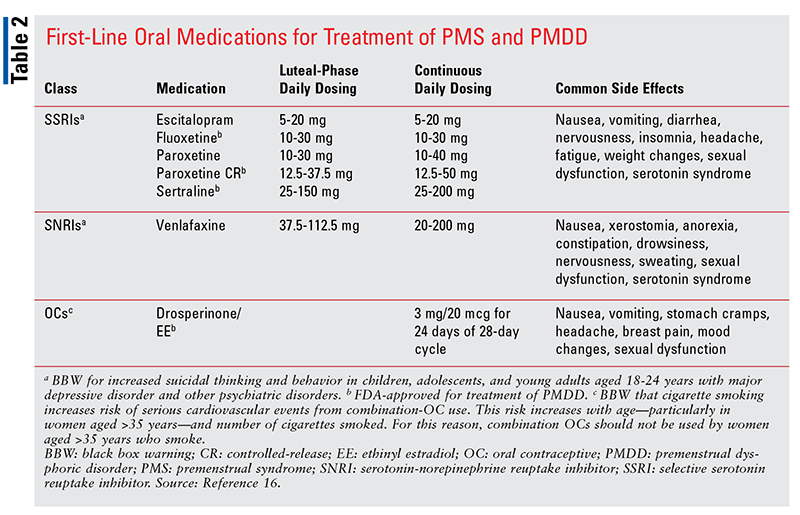
Another first-line option for PMS and PMDD is drospirenone-containing OCs (TABLE 2). For PMDD, the combination OC drospirenone 3 mg and ethinyl estradiol 20 mcg is taken for 24 days of a 28-day cycle.4 This regimen, which is FDA approved for PMDD treatment in women who also desire contraception, has been shown to lessen emotional and physical premenstrual symptoms.7 However, drospirenone-containing OCs carry an increased risk of venous thromboembolism, a serious adverse effect that should be part of the risk-versus-benefit counseling session with the patient.5,6
Patients with PMS or PMDD who experience anxiety, tension, or irritability may benefit from taking the BZD alprazolam during the luteal phase. The use of an intermittent-dosing strategy may reduce the risk of medication misuse. In general, BZDs are less effective than SSRIs in treating symptoms, and they can cause significant central nervous system side effects (e.g., drowsiness, weakness, fatigue, ataxia, and dizziness). Accordingly, alprazolam is considered a second-line treatment option.6,12
For treatment-resistant PMDD, GnRH agonists are a last-resort pharmacologic option.11 GnRH agonists, such as depot leuprolide, have been shown to alleviate physical symptoms as well as some emotional symptoms associated with PMDD.5 However, women with premenstrual depression did not report an improvement in their depression after receiving this treatment.6 Additionally, GnRH agonists may cause hypoestrogen adverse effects including hot flashes, night sweats, and decreased bone density.5,12 For these reasons, combined with the substantial cost of these agents, the use of GnRH agonists is limited.11 Lastly—and only when all other therapeutic options have been exhausted—surgical treatment (hysterectomy with bilateral oophorectomy) may be considered.4,6
The Pharmacist’s Role
The role of the pharmacist in treating PMS and PMDD lies largely in patient education and the dissemination of appropriate information. Because alternative and self-treatment options are often the most accessible therapies for patients with PMS or PMDD, pharmacists frequently interact with patients who are contemplating their use. Pharmacists’ drug expertise is also essential in counseling patients on the proper use of the multitude of prescription medications used to treat symptoms of PMS and PMDD. Further, attention to medication adherence is of utmost importance in ensuring effective pharmacologic therapy results and to reduce the risk of serotonin withdrawal syndrome. Finally, pharmacist knowledge is critical for counseling patients on potential drug interactions and adverse effects of PMS and PMDD treatment options. Patients often assume that supplements are completely safe because of their OTC classification, and that assumption can lead to incorrect dosing and dangerous adverse drug effects or interactions.
Conclusion
PMS and PMDD are common in ovulatory women, and the resultant recurrent symptomology begins during the luteal phase of the menstrual cycle and resolves upon menstruation. These symptoms can cause minor to major impairment that interferes with work, social activities, school, and interpersonal relationships. Pharmacologic treatment options such as SSRIs, SNRIs, BZDs, OCs, and GnRH agonists are available to these patients, as are a number of OTC and alternative therapies. Pharmacists can contribute to positive patient outcomes by guiding medication decisions, providing counseling on both pharmacologic and nonpharmacologic treatments, and supporting the patient through various alternative therapies.
REFERENCES
1. Umland EM, Klootwyk J. Menstruation-related disorders. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 10th ed. New York, NY: McGraw-Hill Education; 2017:1263-1278.
2. DynaMed [online database]. Premenstrual syndrome. www.dynamed.com/condition/premenstrual-syndrome. Accessed June 7, 2021.
3. Hofmeister S, Bodden S. Premenstrual syndrome and premenstrual dysphoric disorder. Am Fam Physician. 2016;94(3):236-240.
4. Yonkers KA, Simoni MK. Premenstrual disorders. Am J Obstet Gynecol. 2018;218(1):68-74.
5. Maharaj S, Trevino K. A comprehensive review of treatment options for premenstrual syndrome and premenstrual dysphoric disorder. J Psychiatr Pract. 2015;21(5):334-350.
6. Appleton SM. Premenstrual syndrome: evidence-based evaluation and treatment. Clin Obstet Gynecol. 2018;61(1):52-61.
7. Lanza di Scalea T, Pearlstein T. Premenstrual dysphoric disorder. Med Clin North Am. 2019;103(4):613-628.
8. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
9. Wittchen HU, Becker E, Lieb R, Krause P. Prevalence, incidence and stability of premenstrual dysphoric disorder in the community. Psychol Med. 2002;32(1):119-132.
10. Potter J, Bouyer J, Trussell J, Moreau C. Premenstrual syndrome prevalence and fluctuation over time: results from a French population-based survey. J Womens Health (Larchmt). 2009;18(1):31-39.
11. Imai A, Ichigo S, Matsunami K, Takagi H. Premenstrual syndrome: management and pathophysiology. Clin Exp Obstet Gynecol. 2015;42(2):123-128.
12. Nevatte T, O’Brien PM, Bäckström T, et al. ISPMD consensus on the management of premenstrual disorders. Arch Womens Ment Health. 2013;16(4):279-291.
13. Green LJ, O’Brien PM, Panay N, Craig M, on behalf of the Royal College of Obstetricians and Gynaecologists. Management of premenstrual syndrome. BJOG. 2017;124(3):e73-e105.
14. Armour M, Ee CC, Hao J, et al. Acupuncture and acupressure for premenstrual syndrome. Cochrane Database Syst Rev. 2018;(8):CD005290.
15. Marjoribanks J, Brown J, O’Brien PM, Wyatt K. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6):CD001396.
16. Lexi-Drugs [online database]. Riverwoods, IL: Lexicomp, Inc. https://.online.lexi.com. Accessed June 30, 2021.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






