US Pharm. 2023;48(7):17-25.
ABSTRACT: Invasive streptococcal disease poses a major health risk, especially for patients at risk for serious disease. The goal of pneumococcal vaccines is to prevent the spread of Streptococcus pneumoniae and invasive disease. Two types of pneumococcal vaccines are currently available in the United States: pneumococcal polysaccharide vaccine (PPSV23) and pneumococcal conjugate vaccine (PCV13, PCV15, and PCV20). The development of new pneumococcal vaccines increases the complexity of recommendations for helping patients keep up-to-date on vaccinations. Pharmacists play a key role in helping determine which vaccines are recommended for individual patients, and they may also be involved in administering these vaccines.
Streptococcus pneumoniae is a gram-positive bacterium typically found in pairs or chains.1 While it is common for S pneumoniae to colonize the respiratory tract, certain individuals are at higher risk for developing pneumococcal disease. Manifestations of pneumococcal disease include noninvasive disease (e.g., acute otitis media, sinusitis) and invasive disease (e.g., pneumonia, meningitis, bacteremia). Patients who contract invasive disease often require hospitalization, and fatality may occur.1 The goal of pneumococcal vaccines is to prevent the spread of S pneumoniae and invasive disease.
Pneumococcal Vaccines
Numerous pneumonia serotypes have been detected in humans; however, only a few of them commonly cause pneumococcal infection.1 Encapsulated S pneumoniae is pathogenic; its capsule is composed of polysaccharides, with its serotypes distinguished based on the composition of the capsular polysaccharides.1 Two types of pneumococcal vaccines are currently available in the United States: pneumococcal polysaccharide vaccine (PPSV23) and pneumococcal conjugate vaccine (PCV13, PCV15, and PCV20).2
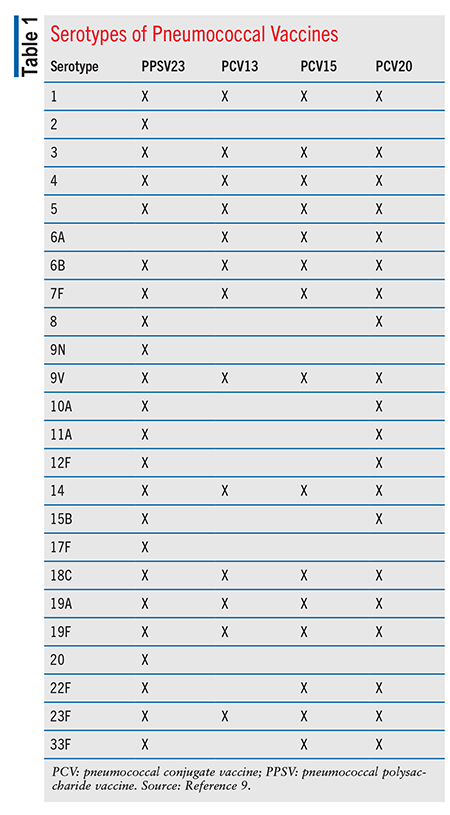
PPSV23: PPSV23, the first pneumococcal vaccine derived from a capsular polysaccharide, consists of 23 capsular polysaccharides that contain 85% to 90% of the isolates for invasive pneumonia in the U.S. (TABLE 1).3 More than 80% of healthy, PPSV23-vaccinated adults develop antibodies to the serotypes in PPSV23.4 Immune response to PPSV23 typically occurs 2 to 3 weeks post vaccination. PPSV23 is less effective for prevention in immunocompromised patients, but even so it is recommended for these patients after prior PCV15 vaccination.4 Children aged <2 years demonstrate a poor immune response to the capsule serotypes in PPSV23, and therefore PPSV23 is not indicated for this population.4,5
PPSV23 (Pneumovax 23) is supplied as a 0.5-mL IM or SC injection.5 Common adverse effects (AEs) of PPSV23 include injection-site soreness/swelling/erythema, headache, asthenia/fatigue, and myalgia. PPSV23 is contraindicated in patients with a history of anaphylaxis from PPSV23 components.5 In adults, PPSV23 may be administered at the same time as an influenza or Shingrix vaccine.6,7 With the exception of Menactra (a meningococcal vaccine), PPSV23 may be administered at the same time as other vaccines in children.7
PCV13: Conjugate vaccines are formulated to attack bacteria with antigens.8 In all of the PCVs (PCV13, PCV15, PCV20), pathogenic pneumonia is encapsulated by a polysaccharide, which protects the antigen and makes it difficult for a young child’s immune system to recognize. PCVs bind polysaccharides to protein antigens to help the immune system develop a response to the capsule.8 The PCV is attached to a carrier protein known as CRM197, a diphtheria-toxin mutation that is nontoxic.9 Attachment to this carrier protein stimulates a protective immune response in children aged <2 years.10
PCV13 (Prevnar 13) contains 13 polysaccharide serotypes of S pneumoniae (TABLE 1).4 This vaccine demonstrated a lower incidence of invasive pneumococcal disease (IPD) from reduced transmission across all age groups and unvaccinated populations.4 PCV13 is supplied as a prefilled syringe containing a single 0.5-mL dose.11 PCV13 is contraindicated in patients with a history of anaphylaxis from PCV13 components or any diphtheria toxoid–containing vaccine. PCV13’s AEs differ by age groups, with varying frequency. Common AEs include (but are not limited to) irritability, injection-site tenderness or swelling, decreased appetite, headache, fever, and alterations in sleep duration. Apnea has occurred after intramuscular vaccination in premature infants; before PCV13 administration, vaccination risks and benefits and the infant’s medical status should be assessed.11 In children, PCV13 may be administered at the same time as other childhood vaccines, with the exception of Menactra.7
PCV15: PCV15 (Vaxneuvance) contains 15 serotypes: the 13 PCV13 serotypes plus serotypes 22F and 33F (TABLE 1).9 PCV15 gained FDA approval in 2021 for use in adults.12 Trials of PCV15 demonstrated similar antibody induction levels and safety as PCV13.4 In 2022, the FDA approved PCV15 for patients aged >6 weeks after safety and comparable antibody induction to PCV13 were demonstrated in patients aged 6 weeks to 17 years.4 PCV15 is supplied as a single-dose 0.5-mL suspension in a prefilled syringe for IM injection.12 Contraindications to PCV15 include history of allergic reaction to PCV15 components or diphtheria toxoid. Common AEs, which differ among age groups, include irritability, somnolence, injection-site pain/swelling/redness, headache, decreased appetite, myalgia, fatigue, and fever.12 In children, PCV15 may be administered with other childhood vaccines, with the exception of Menactra.7 PCV15 may be given at the same time as the influenza vaccine. No data are available for administration at the same time as Shingrix.7
PCV20: PCV20 (Prevnar 20) contains 20 serotypes: the 13 PCV13 serotypes plus serotypes 8, 10A, 11A, 12F, 15B, 22F, and 33F (TABLE 1).9 PCV20 demonstrated safety and similar immunogenicity compared with PCV13.4 PCV20 was FDA approved in 2021 only for patients aged >18 years, and on April 27, 2023, the FDA expanded its approval to children aged >6 weeks.13,14 PCV20 is supplied as a single-dose 0.5-mL suspension in a prefilled syringe for IM injection.13 It is contraindicated in patients with a history of anaphylaxis from PCV20 components or diphtheria toxoid. Common AEs during FDA trials in adults aged 18 to 59 years were injection-site pain, muscle pain, fatigue, headache, and arthralgia and injection-site swelling. Common AEs were similar in adults aged 60 years, although at lower rates.13 PCV20 may be administered at the same time as the influenza vaccine.7 However, no data are available on administration with Shingrix or other vaccines, such as diphtheria and tetanus.7
Trials on the use of PCV20 alone and PCV15 in conjunction with PPSV23 demonstrated these vaccines’ safety and similar immunogenicity, compared with PCV13 alone or in combination with PPSV23, while covering an increased number of serotypes.9 Coverage of more serotypes may reduce cases of pneumococcal disease in adults and increase vaccine coverage among adults, as the new recommendations are simpler. The use of PCV20 alone in adults aged >65 years is predicted to reduce pneumococcal disease occurrence in this age group and demonstrates cost savings in vaccination as well.9
Pediatric Indications
For children aged <2 years, PCV13 or PCV15 is recommended as a series of four doses.2 The first dose should be administered at age 2 months, followed by doses at 4 months and 6 months, and the final dose between age 12 and 15 months. If a child misses this dosing interval, the interval and dose quantity depend on the child’s age when the first dose is administered. The CDC provides an immunization catch-up schedule for further guidance. Children who miss the recommended dosing intervals should still receive vaccinations. It is acceptable to use PCV13 and PCV15 interchangeably.2 Although PCV20 is FDA approved in patients aged >6 weeks, the CDC has yet to make recommendations on its administration in this population.2
Children aged 2 to 5 years with diabetes mellitus, chronic lung disease, cerebrospinal fluid (CSF) leak, chronic heart disease, or a cochlear implant have additional recommendations, as these disease states increase the risk of pneumococcal disease. In these patients, two doses of PCV13 or PCV15 should be administered if the child has an incomplete series of pneumococcal vaccination or is unvaccinated.2 The two doses must be separated by at least 8 weeks. One dose of PCV13 or PCV15 should be administered if three doses of the vaccine series were administered before age 12 months but the fourth dose to complete the four-shot series was not administered. In all scenarios, a PPSV23 vaccination should be administered following completion of PCV vaccination, with the dosing separated by >8 weeks. Children with chronic renal failure/nephrotic syndrome, congenital immunodeficiency, asplenia/splenic dysfunction, HIV, or a disease requiring immunosuppressive or radiation therapy should receive the same vaccines as mentioned above, with the addition of a second PPSV23 vaccination 5 years following the first dose. It is recommended that one dose of PCV13 or PCV15 be given if the child has not yet received a PCV dose. After PCV vaccination, a dose of PPSV23 should be administered >8 weeks after the PCV dose, followed by another PPSV23 dose 5 years after the first PPSV23 dose.2
For children aged 6 to 18 years with CSF leak, cochlear implant, chronic renal failure/nephrotic syndrome, congenital immunodeficiency, asplenia/splenic dysfunction, HIV, sickle cell disease, or a disease requiring radiation therapy or immunosuppressive drugs, one dose of PCV13 or PCV15 is recommended if they have not yet received a PCV vaccine.2 For cochlear-implant and CSF-leak patients, an additional PPSV23 dose should be administered ≥8 weeks after the PCV dose. For the other diseases, two doses of PPSV23 should be given, with the first dose administered 8 weeks after the PCV dose and the second dose administered 5 years after the first PPSV23 dose. In children with diabetes mellitus, chronic heart disease, or chronic lung disease, one dose of PPSV23 should be given if the child did not already receive it earlier.2
Adult Indications
Adults aged 19 to 64 years with chronic diseases that put them at high risk for IPD should receive one dose of PCV15 or PCV20.2 These disease states and risk factors include alcoholism, CSF leak, chronic or congestive heart failure, cardiomyopathy, chronic liver disease, chronic lung disease, chronic obstructive pulmonary disease, asthma, chronic renal failure, cigarette smoking, cochlear implant, congenital or acquired asplenia, congenital or acquired immunodeficiency, diabetes mellitus, generalized malignancy, HIV, Hodgkin’s disease, iatrogenic immunosuppression, leukemia, lymphoma, multiple myeloma, nephrotic syndrome, sickle cell disease, hemoglobinopathy, solid-organ transplant, and immunocompromising conditions.2
If the patient receives a dose of PCV15, a PPSV23 dose should be administered 1 year later.2 A shorter interval of >8 weeks between the PCV15 and PPSV23 doses may be considered in patients with CSF leak, cochlear implant, or an immunocompromising condition.2 This is because these patients are at significantly increased risk for IPD (accounting for >90% of adult IPD cases in 2019).9
These recommendations remain the same for any patient who previously received PCV7 at any time. Patients in this category who received only PPSV23 previously should receive a single dose of PCV15 or PCV20, >1 year after the last PPSV23 dose (FIGURES 1, 2, and 3).2 Patients who received only PCV13 in the past should receive a dose of PCV20 1 year after the last PCV13 dose. An alternative is one dose of PPSV23. The dosing interval between PCV13 and the PPSV23 dose varies based on individual patients’ conditions.2
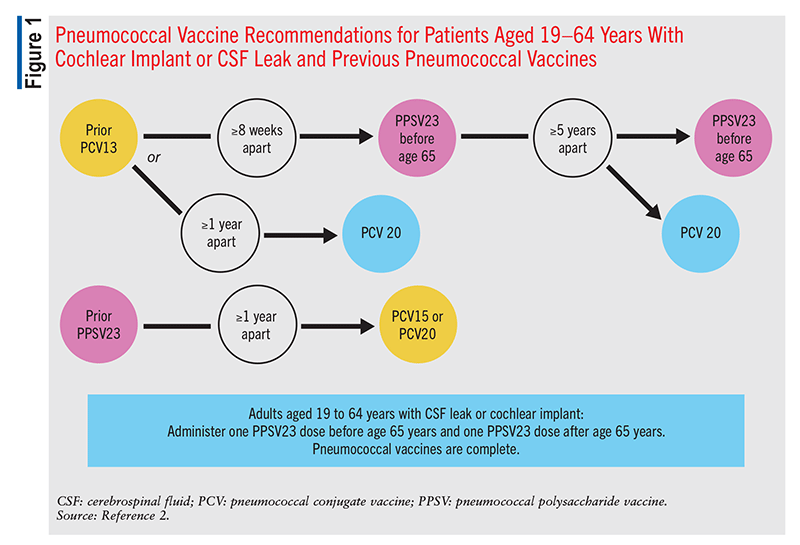
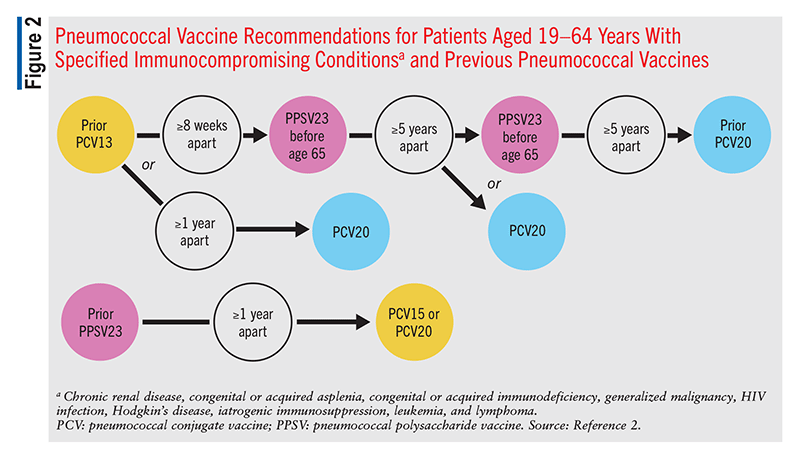
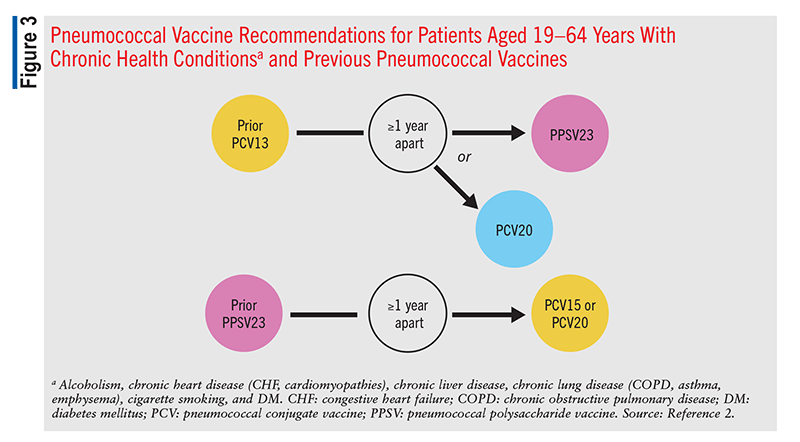
In patients who are immunocompromised, >8 weeks should elapse between PCV13 administration and PPSV23 administration, and these patients may receive two additional doses, with the second dose given 5 years after the first of these (FIGURE 2).2 An 8-week interval may be used in cochlear-implant and CSF-leak patients as well (FIGURE 2). Immunocompromised patients with other chronic conditions should wait >1 year after the last PCV13 dose to receive PPSV23 (FIGURE 3). Patients who have received one dose of both PCV13 and PPSV23 should receive one dose of PCV20 5 years after the last dose of a pneumococcal vaccine. An alternative is to administer a dose of PPSV23 8 weeks after a PCV13 dose or 5 years after the last PPSV23 dose.2
Adults aged >65 years are at increased risk for developing pneumococcal diseases.1 The rate of documented pneumonia infection in the U.S. in patients aged >65 years is 36.4 cases per 100,000, constituting the highest prevalence of any age group. The rate in infants aged <1 year is 34.2 cases per 100,000.1
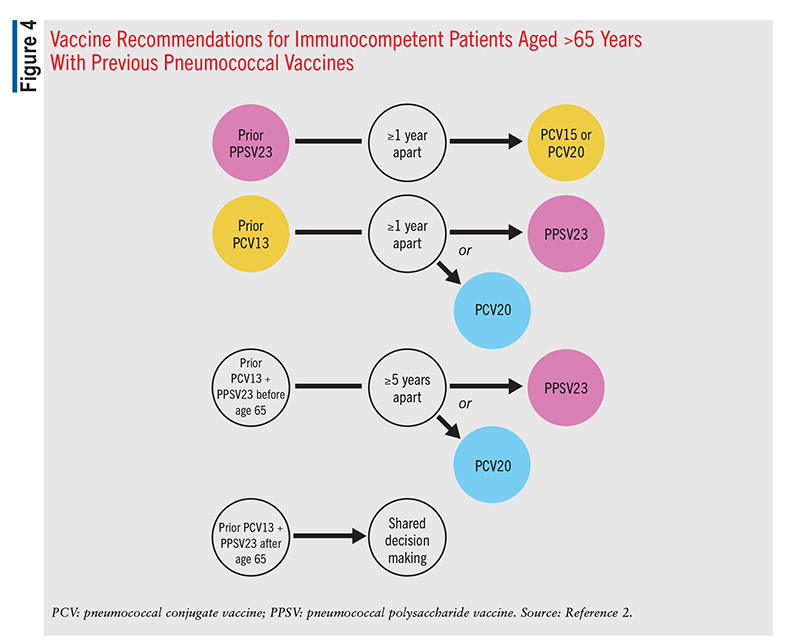
In adults aged >65 years, one dose of PCV15 or PCV20 should be given if the patient has never received a pneumococcal vaccine. If PCV15 is administered, it should be followed by a dose of PPSV23 >1 year after the PCV15 dose.2 PCV20 vaccination does not require a subsequent dose of PPSV23. In patients with a prior history of only a PPSV23 vaccination, one dose of PCV15 or PCV20 should be administered (FIGURE 4). A minimum 1-year interval between the PPSV23 dose and the PCV dose is recommended. A PPSV23 dose is not indicated following the PCV dose. For patients aged >65 years who have received only PCV13, one dose of PCV20 is recommended 1 year after the last PCV13 dose. An alternative is to administer a single dose of PPSV23 1 year after the last PCV13 dose (FIGURE 4). If a patient has received both PCV13 and PPSV23 before age 65 years, one dose of PCV20 >5 years after the last pneumococcal dose is recommended. In patients receiving a PPSV23 vaccine, an 8-week interval since the last PCV dose may be used in those with a cochlear implant, CSF leak, or an immunocompromised state.2 In patients who received both PCV13 and PPSV23 after age 65 years, it may be appropriate to administer a dose of PCV20. The decision is made on a case-by-case basis using shared clinical decision making (FIGURE 4). The PCV20 dose should be administered >5 years after the patient’s last pneumococcal dose.2
The Pharmacist’s Role
The development of new pneumococcal vaccines increases the complexity of the recommendations provided to help providers and patients keep up-to-date on patients’ vaccinations. Pharmacists can make recommendations for appropriate pneumococcal vaccinations based on a patient’s risk factors for pneumococcal disease and previous history of vaccination. Pharmacists who can legally administer vaccinations have the additional responsibility of screening patients and administering the recommended vaccines. For guidance on which pneumococcal vaccines to recommend, pharmacists may use the CDC’s mobile app, PneumoRecs VaxAdvisor.15
REFERENCES
1. Dion CF, Ashurst JV. Streptococcus pneumoniae. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-.
2. CDC. Pneumococcal vaccination: summary of who and when to vaccinate. www.cdc.gov/vaccines/vpd/pneumo/hcp/who-when-to-vaccinate.html. Accessed May 1, 2023.
3. Tereziu S, Minter DA. Pneumococcal vaccine. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-.
4. CDC. About pneumococcal vaccines. www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html. Accessed May 1, 2023.
5. Pneumovax 23 (pneumococcal vaccine polyvalent) product information. Rahway, NJ: Merck Sharp & Dohme LLC; April 2023.
6. CDC. Administering Shingrix. www.cdc.gov/vaccines/vpd/shingles/hcp/shingrix/administering-vaccine.html. Accessed May 1, 2023.
7. CDC. Administering pneumococcal vaccines. www.cdc.gov/vaccines/vpd/pneumo/hcp/administering-vaccine.html. Accessed May 1, 2023.
8. CDC. Understanding how vaccines work. www.cdc.gov/vaccines/hcp/patient-ed/conversations/downloads/vacsafe-understand-bw-office.pdf. Accessed May 31, 2023.
9. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among U.S. adults: updated recommendations of the Advisory Committee on Immunization Practices–United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):109-117.
10. Daniels CC, Rogers PD, Shelton CM. A review of pneumococcal vaccines: current polysaccharide vaccine recommendations and future protein antigens. J Pediatr Pharmacol Ther. 2016;21(1):27-35.
11. Prevnar 13 (pneumococcal 13-valent conjugate vaccine) product information. Philadelphia, PA: Wyeth Pharmaceuticals LLC; July 2019.
12. Vaxneuvance (pneumococcal 15-valent conjugate vaccine) product information. Whitehouse Station, NJ: Merck & Co, Inc; June 2022.
13. Prevnar 20 (pneumococcal 20-valent conjugate vaccine) product information. Philadelphia, PA: Wyeth Pharmaceuticals LLC; April 2023.
14. Pfizer Inc. U.S. FDA approves PREVNAR 20®, Pfizer’s 20-valent pneumococcal conjugate vaccine for infants and children. www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-prevnar-20r-pfizers-20-valent-pneumococcal. Accessed May 1, 2023.
15. CDC. PneumoRecs VaxAdvisor mobile app for vaccine providers. www.cdc.gov/vaccines/vpd/pneumo/hcp/pneumoapp.html. Accessed May 1, 2023.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





