US Pharm. 2007;32(9):HS-3-HS-15.
Polycystic
ovary syndrome (PCOS) is a heterogeneous metabolic and reproductive disorder
characterized by androgen excess and menstrual irregularities.1-3
Insulin resistance and obesity are also common components of the syndrome.
1,2 PCOS is one of the most prevalent endocrine disorders in women,
affecting an estimated 4% to 10% of females of reproductive age.1,2,4
Anovulation associated with PCOS is a leading cause of infertility; however,
complications of PCOS extend beyond fertility concerns. Possible long-term
consequences of PCOS include an increased risk of endometrial hyperplasia or
cancer, metabolic syndrome, type 2 diabetes, and sleep apnea.1,4
Because of the potential for significant consequences, appropriate
identification and treatment of individuals with PCOS are essential.
Pathophysiology
The primary
pathophysiologic defect in PCOS remains unknown.1,2 Key features of
the syndrome include altered gonadotropin secretion, hyperandrogenism, and
insulin resistance. Women with PCOS have an increased pulse frequency of the
gonadotropin luteinizing hormone (LH). It is uncertain whether increased LH
release is due to an inherent defect in the pulsatile release of
gonadotropin-releasing hormone (Gn-RH) from the hypothalamus or to low levels
of circulating progestin, as lower levels of progestin may be associated with
accelerated Gn-RH pulsatility.1,2 Regardless of the source,
increased pulsatility of Gn-RH favors the production of LH over
follicle-stimulating hormone (FSH) and results in relatively higher
circulating levels of LH. The effect of higher concentrations of LH is
increased production of androgens by ovarian theca cells.1,2 The
combination of androgen excess and insufficient FSH may lead to anovulation.
When ovulation does not occur for a prolonged period, the polycystic ovary may
result.3
In addition to
hyperandrogenism and altered gonado-tropin dynamics, insulin resistance is a
prominent characteristic of PCOS. Hyperinsulinemia resulting from insulin
resistance is strongly correlated with androgen excess in PCOS.5 In
the ovary, insulin works synergistically with LH to stimulate androgen
production.1,2 Insulin also reduces circulating levels of sex
hormone–binding globulin (SHBG), a glycoprotein that binds testosterone.
1,5 Thus, the relative proportion of free, or biologically active,
testosterone is increased.
Clinical Presentation
PCOS is
characterized by a broad spectrum of biochemical and clinical manifestations.
Key features are menstrual irregularities and androgen excess. Menstrual
irregularities, such as oligo-ovulation or anovulation, present as
oligomenorrhea (fewer than nine menses per year) or amenorrhea (absence of
menses).1,6,7 Menarche may occur early or at the normal pubertal
onset, and oligomenorrhea typically ensues shortly thereafter.6
Anovulation, leading to dysfunctional uterine bleeding and infertility, is a
leading cause for women with PCOS to seek medical attention. It is estimated
that amenorrhea is present in 50% of cases, while dysfunctional uterine
bleeding is present in 30%.8,9 Clinical manifestations of androgen
excess or hyperandrogenism include hirsutism, acne, and male-pattern hair
loss. Hirsutism, occurring in approximately 70% of PCOS cases, is commonly
noted on the upper lip, chin, periareolar area, and along the linea alba of
the lower abdomen.8,9 Patients may have biochemical evidence of
androgen excess represented by elevated testosterone levels.1,6,7
Women with PCOS are at an increased
risk of endometrial cancer related to ongoing exposure of the endometrium to
unopposed estrogen. This requires continuing surveillance for endometrial
hyperplasia by ultrasound, biopsy, or hysteroscopy.7,10 In
addition, women with the syndrome require long-term medical care, as they are
at a high risk of a number of chronic metabolic and cardiovascular
abnormalities.1,9 Affected women may have metabolic disturbances,
including insulin resistance, hyperinsulinemia, dyslipidemia, and obesity.
9,11 The exact mechanism behind the insulin resistance is not fully
elucidated. A study by Dunaif et al. demonstrated that 50% of PCOS patients
have insulin resistance and compensatory hyperinsulinemia that is not due to
obesity.11 Long-term studies have found that women with PCOS have a
three- to seven-times higher risk of type 2 diabetes.7,9
Diagnosis
PCOS was first
described in 1935 in a series of case reports published by Stein and
Leventhal. This research was the first association between the presence of
polycystic ovaries, amenorrhea, and hyperandrogenism.12 Since 1935,
the definition of PCOS has continued to change and remains controversial.
7,13 In 2003, an international consensus group convened to develop
standardized diagnostic criteria for PCOS.7 According to this
expert panel, PCOS is present when a patient meets at least two of the
following three criteria: oligo-ovulation or anovulation, clinical and/or
biochemical hyperandrogenism, and polycystic ovaries defined by ultrasound.
Other disorders with similar clinical presentations, such as Cushing's
syndrome, androgen-secreting tumors, and congenital adrenal hyperplasia, must
be ruled out prior to the diagnosis of PCOS.7
Treatments
There are a number
of therapeutic interventions available for the treatment of PCOS, including
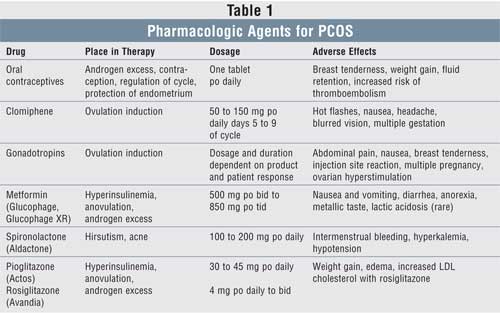
lifestyle modification, surgery, and pharmacologic therapy (see TABLE 1
). Treatment goals include alleviation of symptoms, restoration of fertility,
and prevention of long-term complications. Prior to the initiation of
therapeutic interventions, it is essential to determine whether the patient is
planning to become pregnant in the near future, as this will be a major
determinant in the choice of therapy.6,10
Nonpharmacologic:
Lifestyle modification is critical to prevent or delay serious health
consequences associated with PCOS, and it should be initiated in all
overweight women with the syndrome. Diet and exercise are two of the best
initial options to restore fertility and alleviate symptoms in obese women
with PCOS.10 Clinical trials suggest that a diet low in saturated
fats and high in dietary fiber with low-glycemic-index carbohydrates is
preferred.14 A study conducted by Huber-Buchholz et al. evaluated
the effect of a six-month diet and exercise program in 28 obese women with
PCOS who were trying to become pregnant or restore menstrual regularity.15
Study results demonstrated that with a mean weight loss of 2% to 5%, women
were able to reduce central fat by 11%, improve their insulin sensitivity
index by 71%, reduce fasting insulin levels by 33%, and reduce LH by 39%. Of
the 15 anovulatory women enrolled in the study, nine regained regular
ovulation and two became pregnant.15 If lifestyle modification
fails to reach treatment goals, pharmacologic agents may be used.
Hirsutism is commonly managed
with cosmetic hair removal procedures. Women may elect to use laser hair
removal, electrolysis, bleaching, depilatory agents, waxing, or shaving.
4,10 When performing a patient interview, it is important to inquire
about a history of excessive, male-pattern hair growth, as hirsutism may be
improved or absent due to cosmetic removal.
An additional therapeutic
intervention that may be used for ovulation induction is the surgical
procedure laparoscopic ovarian drilling, although its use has declined since
the 1990s with the introduction of equally effective pharmacologic agents.
6,16
Oral Contraceptives:
When contraception is desirable, combination oral contraceptives provide an
effective option for managing both the irregular menses and the androgen
excess associated with PCOS. The estrogen component of oral contraceptives
suppresses secretion of LH, resulting in a reduction in ovarian androgen
production.1,17 Estrogen also promotes the synthesis of SHBG,
leading to a decrease in free circulating testosterone. Both of these actions
help target the acne and the hirsutism seen in PCOS. The progestin component
of oral contraceptives protects the endometrium from the effects of unopposed
estrogen. Thus, in addition to providing monthly withdrawal bleeding and
regulation of the menstrual cycle, use of oral contraceptives decreases the
risk of endometrial hyperplasia and cancer.4,17 Women who are
unable to tolerate combination oral contraceptives may achieve cyclic
withdrawal bleeding and endometrial protection with intermittent
administration of a progestin such as medroxyprogesterone.4,17
When choosing a combination
oral contraceptive for patients with PCOS, oral contraceptives containing
progestins with low androgenicity are recommended to avoid potentiating
existing androgen excess. In that regard, progestins with minimal androgenic
activity, such as desogestrel or norgestimate, are preferred.1,17
Drospirenone, the newest progestin, has both antiandrogenic and
antimineralocorticoid activity.18 Initial studies have demonstrated
beneficial effects of drospirenone on acne and hirsutism in patients with PCOS.
19,20 In general, use of combination oral contraceptives containing the
more androgenic progestins, such as levonorgestrel and norgestrel, is not
recommended in this patient population.
One concern with the use of
oral contraceptives in PCOS is the potential for unfavorable effects on
insulin resistance.1,17 Oral contraceptives containing progestins
with higher androgenic activity are associated with increased insulin
resistance. Conflicting evidence exists as to whether the less androgenic
progestins have significant effects on insulin sensitivity.
Clomiphene:
In patients who want to conceive, weight loss is the recommended initial
strategy to promote fertility. For patients who are unable to achieve
resumption of ovulatory cycles with modest weight loss, or for lean patients
with PCOS-related infertility, clomiphene citrate is a preferred pharmacologic
therapy for ovulation induction.16 Clomiphene is a nonsteroidal
estrogen receptor modulator that blocks negative feedback of estrogen on the
hypothalamus. The result is an increase in secretion of FSH and LH from the
pituitary and subsequent promotion of follicular growth and maturation.10
A usual starting dose of clomiphene is 50 mg per day on days 5 to 9 of the
cycle. At this dose, about 50% of women will ovulate, and with higher doses,
an additional 25% to 30% will ovulate.16 Patients receiving
clomiphene should be counseled regarding the potential for multiple gestation
and adverse effects such as hot flashes, nausea, and headache.
For women who do not respond
to higher doses of clomiphene, the addition of metformin has been shown to
improve the ovulatory response rate.21,22 However, results of a
recent randomized, controlled trial failed to show the benefit of
extended-release metformin plus clomiphene over clomiphene alone on the
live-birth rate.23 The addition of dexamethasone to clomiphene has
also been shown to be effective in clomiphene-resistant patients with PCOS.
24 In 80 women with PCOS, dexamethasone 2 mg daily (days 3-12 of cycle)
plus clomiphene 100 mg daily (days 3-7 of cycle) was compared to clomiphene
alone. The dexamethasone group experienced significantly higher rates of
ovulation (75% vs. 15%) and subsequent pregnancy (40% vs. 5%). The mechanism
of the beneficial effects of dexamethasone has not been fully elucidated, but
it is thought to be related to decreased levels of free testosterone and LH
and enhancement of follicular development.25
Gonadotropin Therapy:
Women who do not respond
to clomiphene or the combination of clomiphene plus metformin or dexamethasone
may be candidates for ovulation induction with gonadotropins.16
Low-dose recombinant human FSH is injected daily and titrated carefully until
a desired follicular response is achieved. In general, women with PCOS do not
require exogenous LH, since LH levels are usually elevated. Ovarian response
to FSH is monitored via ultrasound, and when the appropriate follicular size
is reached, human chorionic gonadotro pin (hCG) is administered to
stimulate final follicular maturation and ovulation.10,16
Gonadotropin is often used in conjunction with in vitro fertilization and
should be administered only under the guidance of a trained infertility
specialist. Potential adverse effects of gonadotropin include nausea, breast
tenderness, multiple gestation, and ovarian hyperstimulation syndrome.
Metformin:
Metformin, a biguanide, is FDA approved for use as an oral hypoglycemic agent
in type 2 diabetes. It is used off-label in PCOS to treat hyperinsulinemia,
anovulation, and androgen excess.1,17 The primary mechanism of
action for metformin is reduction in hepatic glucose production. In addition,
metformin improves peripheral insulin sensitivity, decreases intestinal
glucose absorption, decreases lipolysis, and may act directly to diminish
ovarian steroid production.17 It is believed that in women with
PCOS, the direct action on ovarian steroid production is not the primary
reason for diminished ovarian androgen production. Rather, it is the reduction
in hepatic glucose production, and therefore a lower insulin concentration,
that is thought to be responsible for reduced androgen production in ovarian
theca cells.1
A recent meta-analysis
reviewed 13 clinical trials involving metformin treatment in 543 women with
PCOS.26 It was determined that women taking metformin had an odds
ratio for ovulation of 3.88 (95% confidence interval [CI], 2.25-6.69) compared
with placebo, and those taking metformin plus clomiphene compared with
clomiphene alone had an odds ratio for ovulation of 4.41 (95% CI, 2.37-8.22).
Metformin was found to have a significant effect in reducing fasting insulin
levels, blood pressure, and low-density lipoprotein cholesterol, with no
evidence of an effect on body mass index or waist-to-hip ratio. Women taking
metformin had significantly more nausea and vomiting and gastrointestinal
disturbance compared to placebo; no serious adverse effects were reported in
this analysis.26
Metformin may have beneficial
effects in the improvement of androgen excess, although it is not as effective
as oral contraceptives and antiestrogens. Limited data are available at this
time to recommend metformin for management of hirsutism.10,17
Common adverse effects of
metformin include nausea and vomiting and diarrhea, which typically diminish
over time. Lactic acidosis is a rare adverse event, and metformin should not
be prescribed for women with conditions that increase this risk (i.e., kidney
or liver disease, alcoholism, treatment for heart failure). Routine monitoring
of serum creatinine is recommended, since metformin is contraindicated in
women with a serum creatinine greater than or equal to 1.4 mg/dL.16,17
Metformin is an effective
agent to induce ovulation in women with PCOS, and it should be considered a
first-line agent. Prior to initiation of therapy, women should be counseled on
the effects in pregnancy. If a woman is started on metformin therapy and she
is not seeking to become pregnant, contraceptives should be initiated.1,17
Metformin is not FDA approved for use in pregnancy; nevertheless, many women
with PCOS take this medication for ovulation induction and risk exposure to
the fetus. Currently there are no specific neonatal complications reported in
women taking metformin during pregnancy, and it is listed as pregnancy
category B.10
Antiandrogens:
Spironolactone, an aldosterone antagonist, is the most commonly used
antiandrogen for the treatment of hirsutism in PCOS. Use in PCOS as
monotherapy or in combination with oral contraceptives is currently not
approved by the FDA.17 Spironolactone reduces androgen production
by inhibition of the androgen receptor and the enzyme 5-alpha-reductase.10
Based on very few studies, spironolactone has shown subjective improvement in
hirsutism in women with PCOS.27 Flutamide is another antiandrogen,
but its use is limited secondary to hepatotoxicity.17
Adverse effects of
spironolactone include intermenstrual bleeding, hyperkalemia, and hypotension.
Women taking spironolactone should have yearly renal and liver function tests
and should have electrolytes monitored annually. Spironolactone is pregnancy
category C and should be avoided in women seeking pregnancy.
Thiazolidinediones:
Pioglitazone and rosiglitazone, members of the thiazolidinedione class (TZDs),
are FDA approved as oral antidiabetic agents for the treatment of type 2
diabetes. Thiazolidinediones may be beneficial in PCOS to treat
hyperinsulinemia, anovulation, and androgen excess. The primary mechanism of
action is improved insulin sensitivity in the liver, adipose tissue, and
skeletal muscle. As with metformin, the TZDs may have a direct effect on
ovarian steroid production, although it is thought that the reduction in
insulin levels is primarily responsible for decreased androgen concentrations.
1 Only small studies evaluating the use of TZDs in PCOS have been
published at this time, but the results appear to have clinical significance.
A randomized, double-blind,
placebo-controlled trial evaluating the effect of pioglitazone 30 mg daily for
three months found that despite significant weight gain, pioglitazone
significantly improved insulin sensitivity, androgen excess, and ovulation
rates in women with PCOS compared to placebo.28 A trial involving
30 overweight women with PCOS randomly assigned to rosiglitazone or placebo
for three months reported significant improvements in menstrual cyclicity, a
decrease in serum androgen levels, and an improvement in glucose tolerance and
insulin resistance.29
There is a greater concern for
the effects of thiazolidinediones on the fetus compared with metformin. Animal
studies have shown that TZDs cause growth retardation in mid- to late
gestation. Pioglitazone and rosiglitazone, classified as pregnancy category C,
should not be used in women who are trying to become pregnant.17
TZDs are a viable treatment option
in women who are not seeking to become pregnant, but they should be used with
caution due to limited clinical data in PCOS and the potential for weight
gain. A recent meta-analysis of 42 trials involving rosiglitazone for treating
type 2 diabetes found an increased risk of myocardial infarction and death
from cardiovascular causes.30 Future studies are needed to confirm
cardiovascular risks with rosiglitazone.
Severe hepatotoxicity
observed with troglitazone has not been seen with pioglitazone and
rosiglitazone, but initial and periodic evaluation of liver function is still
recommended.17
Pharmacist's Role
Pharmacists can
have an integral role in optimizing drug therapy outcomes for patients with
PCOS. Of primary importance, pharmacists need to recognize that medications
such as metformin and the thiazolidinediones may be prescribed for PCOS. By
being aware of this indication, pharmacists can avoid inadvertently counseling
patients that they are receiving a prescription for the treatment of type 2
diabetes. As with other prescriptions, pharmacists should provide counseling
on appropriate use and expected effects of medications prescribed for PCOS.
For example, patients receiving a prescription for oral contraceptives should
receive counseling on appropriate administration, importance of adherence, and
potential adverse effects such as breast tenderness, breakthrough bleeding,
weight gain, and fluid retention. Pharmacists should also screen for potential
drug interactions with medications for PCOS; e.g., certain antibiotics with
oral contraceptives. In addition, pharmacists can make a significant impact on
patients with PCOS by providing additional information about the disease,
counseling on long-term consequences, and encouraging adherence to therapy to
minimize risks of metabolic syndrome, type 2 diabetes, and endometrial cancer.
Conclusion
PCOS is a complex
endocrine and metabolic disorder characterized by androgen excess,
oligo-ovulation or anovulation, and, in the majority of cases, insulin
resistance. Acne, hirsutism, irregular menses, and infertility are common
reasons that women with PCOS seek treatment. Depending on presenting symptoms
and the desire for conception, PCOS may be managed with a variety of
medications, including oral contraceptives, metformin, thiazolidinediones,
spironolactone, and agents for ovulation induction. Patients with PCOS are at
risk for chronic complications such as endometrial hyperplasia, metabolic
syndrome, and type 2 diabetes. Through patient education and collaboration
with other health care providers, pharmacists can play a key role in reducing
the long-term health risks associated with PCOS.
References
1. Ehrmann DA.
Polycystic ovary syndrome. N Engl J Med. 2005;352:1223-1236.
2. Guzick DS.
Polycystic ovary syndrome. Obstet Gynecol. 2004;103:181-193.
3. Hunter MH, Sterrett
JJ. Polycystic ovary syndrome: it's not just infertility. Am Fam
Physician. 2000;62:1079-1088, 1090.
4. Setji TL, Brown AJ.
Polycystic ovary syndrome: diagnosis and treatment. Am J Med.
2007;120:128-132.
5. Livingstone C,
Collison M. Sex steroids and insulin resistance. Clin Sci.
2002;102:151-166.
6. Carr BR, Bradshaw
KD. Disorders of the ovary and female reproduction tract. In: Kasper DL, Fauci
A, et al, eds. Harrison's Principles of Internal Medicine. Volume
2. 16th ed. New York, NY: McGraw-Hill; 2005:2204-2205.
7. Rotterdam
ESHRE/ASRM-sponsored PCOS consensus workshop group 2004. Revised 2003
consensus on diagnostic criteria and long-term health risks related to
polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41-47.
8. Goldzieher JW,
Axelrod LR. Clinical and biochemical features of polycystic ovarian disease.
Fertil Steril. 1963;14:631-653.
9. Vignesh JP, Mohan V.
Polycystic ovary syndrome: a component of metabolic syndrome? J Postgrad Med
. 2007;53:128-134.
10. Stankiewicz M,
Norman R. Diagnosis and management of polycystic ovary syndrome: a practical
guide. Drugs. 2006;66:903-912.
11. Dunaif A, Segal K,
et al. Profound peripheral insulin resistance independent of obesity, in
polycystic ovary syndrome. Diabetes. 1989;38:1165-1174.
12. Stein IF, Leventhal
ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet
Gynecol. 1935;29:181-191.
13. Franks S.
Controversy in clinical endocrinology: diagnosis of polycystic ovarian
syndrome: in defense of the Rotterdam Criteria. J Clin Endocrinol Metab
. 2006;91:786-789.
14. Hoeger KM. Obesity
and lifestyle management in polycystic ovary syndrome. Clin Obset Gynecol
. 2007;50:277-294.
15. Huber-Buchholz MM,
Carey D, Norman RJ. Restoration of reproductive potential by lifestyle
modification in obese polycystic ovary syndrome: role of insulin sensitivity
and luteinizing hormone. J Clin Endocrinol Metab. 1999;84:1470-1474.
16. Guzick D. Ovulation
induction management of PCOS. Clin Obstet Gynecol. 2007;50:255-267.
17. Dronavalli S,
Ehrmann D. Pharmacologic therapy for polycystic ovary syndrome. Clin Obstet
Gynecol. 2007;50:244-254.
18. Yaz [package
insert]. Wayne, NJ: Bayer HealthCare Pharmaceuticals Inc. February 2007.
19. Guido M, Romualdi
D, et al. Drospirenone for the treatment of hirsute women with polycystic
ovary syndrome: a clinical endocrinological, metabolic pilot study. J Clin
Endocrinol Metab. 2004;89:2817-2823.
20. Palep-Singh M, Mook
K, et al. An observational study of Yasmin in the management of women with
polycystic ovary syndrome. J Fam Plann Reprod Health Care.
2004;30:163-165.
21. Nestler JE,
Jakubowicz DJ, et al. Effects of metformin on spontaneous and
clomiphene-induced ovulation in the polycystic ovary syndrome. New Engl J
Med. 1998;338:1876-1880.
22. Vandermolen DT,
Ratts VS, et al. Metformin increases the ovulatory rate and pregnancy rate
from clomiphene citrate in patients with polycystic ovary syndrome who are
resistant to clomiphene citrate alone. Fertil Steril. 2001;75:310-315.
23. Legro RS, Barnhart
HX, et al. Clomiphene, metformin, or both for infertility in the polycystic
ovary syndrome. N Engl J Med. 2007;356:551-566.
24. Elnashar A,
Abdelmageed E, et al. Clomiphene citrate and dexamethasone in treatment of
clomiphene citrate-resistant polycystic ovary syndrome: a prospective,
placebo-controlled study. Hum Reprod. 2006;21:1805-1808.
25. Parsanezhad ME,
Alborzi S, et al. Use of dexamethasone and clomiphene citrate in the treatment
of clomiphene citrate-resistant patients with polycystic ovary syndrome and
normal dehydroepiandrosterone sulfate levels: a prospective, double-blind,
placebo-controlled trial. Fertil Steril. 2002;78:1001-1004.
26. Lord JM, Flight IH,
et al. Insulin-sensitizing drugs (metformin, troglitazone, rosiglitazone,
pioglitazone, D-chiro-inositol) for polycystic ovary syndrome (Cochrane
Review). In: The Cochrane Library. 2007;2:CD003053.
27. Christy NA, Franks
AS, Cross LB. Spironolactone for hirsutism in polycystic ovary syndrome.
Ann Pharmacother. 2005;39:1517-1521.
28. Brettenthaler N,
Geyter C, et al. Effect of the insulin sensitizer pioglitazone on insulin
resistance, hyperandrogenism, and ovulatory dysfunction in women with
polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:3835-3840.
29. Rautio K,
Tapanainen JS, et al. Endocrine and metabolic effects of rosiglitazone in
overweight women with PCOS: a randomized placebo-controlled study. Hum
Reprod. 2006;21:1400-1407.
30. Nissen SE, Wolski
K. Effect of rosiglitazone on the risk of myocardial infarction and death from
cardiovascular causes. N Engl J Med. 2007;356:2457-2471.
To comment on this article, contact
editor@uspharmacist.com.





