US
Pharm. 2006;3:33-47.
During the past 20 years,
more medications have been made available without a prescription than ever
before.1 Despite the large number of patients who self-treat, only
a small percentage seek the advice of a health care professional when
selecting a product.2,3 This presents a problem, considering the
size of this potential patient population. This population includes many
patients who have chronic health conditions, which can be worsened by the
inappropriate use of OTC medications. Of particular concern is the safe use of
nonprescription medications in pregnant women. A recent study showed that
during pregnancy, 92.6% and 45.2% of women utilize OTC and herbal medications,
respectively. Analgesics and cough and cold preparations are two of the most
common categories of OTC products purchased during pregnancy.4
Safety Data and Pregnancy
At this time,
there are limited safety data on the use of OTC medications during pregnancy.
Due to ethical concerns, most safety data available have been provided by
postmarketing surveillance reports and retrospective studies. The FDA has
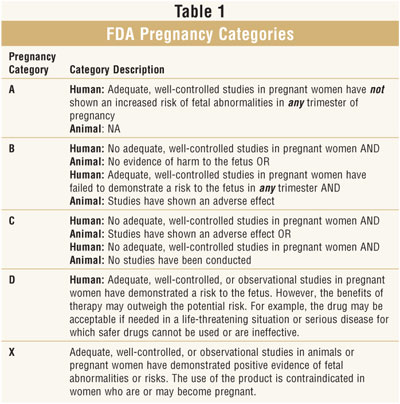
developed pregnancy categories for both OTC and prescription medications. This
classification system allows practitioners to make educated decisions about
the use of medications during pregnancy. The system is organized into five
categories: A, B, C, D, and X. Each letter indicates the level of safety
evidence available to support the use of a medication during pregnancy (table 1
).5,6 The safety of a medication during pregnancy is often
dependent on the trimester or stage of fetal embryonic development.
Benefits Versus Risks
In the United
States, about 150,000 babies are born each year with birth defects.7
Birth defects can occur due to many nonpharmacologic factors. Some of the
most common defects are spina bifida, microtia, hypoplastic left heart, cleft
palate, cleft lip, esophageal atresia, anencephaly, omphalocele, and limb
reduction.7 Practitioners must weigh the benefits versus the risks
when recommending OTC analgesics and cough and cold preparations to pregnant
women. Since ailments treated with OTC and herbal products in pregnant women
are not usually life-threatening, practitioners should also consider
suggesting nonpharmacologic remedies, such as rest and fluids.
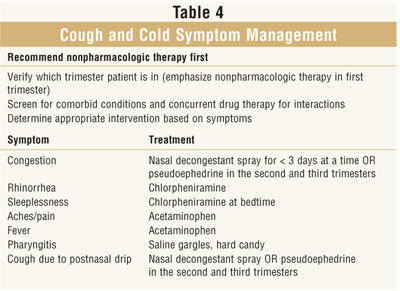
This article presents
information on some common OTC analgesic and cough and cold preparations
available. Each section discusses the product, pregnancy category, information
regarding safety data in pregnancy, dosing, side effects, and
contraindications. The comparison of risks and benefits must be considered for
each individual patient. Information relating to when patients should refer to
a physician (Tables 2 and 3) is included to assist with the decision-making
process.

Analgesics
Acetaminophen:
During pregnancy, acetaminophen is the most widely recommended analgesic
medication. Acetaminophen is pregnancy category B during all three trimesters,
making it the pain reliever of choice for pregnant patients.8
Acetaminophen does appear to cross the placenta, but three studies that
involved over 10,000 newborn infants have shown no increased risk of
malformations in newborns exposed to acetaminophen in the first trimester.
9 One small, retrospective study showed a slightly higher incidence of
gastroschisis (a birth defect resulting in bowel protrusion near the umbilical
cord) in newborns who had been exposed to the drug. The risk of gastroschisis
in the infant was higher in mothers who had taken acetaminophen in conjunction
with pseudoephedrine.10 Some published case reports have cited
acetaminophen exposure as the possible cause for adverse effects, including
one case of fatal kidney disease, but these reports are rare.9

Overall, acetaminophen is used
extensively during pregnancy, and few adverse effects have been reported.
Patients can be advised to take 325 to 1,000 mg every four to six hours as
needed (maximum of 4,000 mg/day). Pregnant patients should be instructed to
use the smallest effective dose of the medication. If the medication is
ineffective, or required use is more than 10 days, the patient should be
referred to her physician. Other pregnant women who should consult a physician
before starting self-treatment are those with renal or hepatic dysfunction, a
high-risk pregnancy, a complaint of headache in the third trimester (a
possible sign of increased blood pressure and eclampsia), any pain rated
higher than 6 on a scale of 1 through 10, presence of fever or other signs of
infection, or pain associated with any type of trauma.11
Nonpharmacologic recommendations can be made according to the type of pain.
For example, a patient complaining of headache should try resting and lying
down in a dark, quiet room.
NSAIDs:
Nonsteroidal anti-inflammatory drugs (NSAIDs) that are available without a
prescription include ibuprofen, naproxen, and ketoprofen. All three are
pregnancy category B in the first and second trimester, and category D in the
third trimester. The most studied NSAID in pregnancy is the prescription
product indomethacin. Similar to the OTC products, indomethacin is also a
pregnancy category B in the first trimester and D in the third trimester. The
data for indomethacin could be applied to the entire class of NSAIDs, as
studies for other drugs in this class are lacking.8 Compared to
acetaminophen, NSAIDs have been linked with an increased risk of gastroschisis
at a slightly higher rate.10 In addition, all NSAIDs used near term
are associated with oligohydramnios (a low level of amniotic fluid), a
premature closure of the ductus arteriosus, and inhibition of labor.9
Unfortunately, complications can also result in the newborn, such as
pulmonary hypertension, fetal nephrotoxicity, and periventricular hemorrhage.
8
Generally, NSAIDs should not
be used during pregnancy without approval from the patient's physician.
However, when patients require self-treatment with NSAIDs, appropriate doses
can be recommended: 200 to 400 mg of ibuprofen every four to six hours
(maximum 1,200 mg/day); 220 mg of naproxen every eight to 12 hours (maximum
660 mg/day); and 12.5 mg of ketoprofen every six to eight hours, repeating the
initial dose after one hour if no effect (maximum 75 mg/day).11
Whenever possible, the
smallest effective dose should be used. The patient should be screened and
referred to her physician when appropriate. Appropriate referrals include, but
are not limited to, the criteria mentioned for acetaminophen, a history of
gastrointestinal ulceration, blood pressure problems, and a history of
NSAID-sensitive asthma. Pregnant patients should not take NSAIDs for longer
than 48 hours without contacting their physician.
Salicylates
Aspirin is a
pregnancy category C in doses less than 150 mg daily and a category D in
standard doses in all three trimesters.9 Salicylates have been
associated with increased mortality, neonatal hemorrhage, decreased birth
weight, prolonged gestation and labor, and possible birth defects. A pregnant
patient should never take aspirin without the approval and guidance of her
physician.
Decongestants
Oral:
Pseudoephedrine and phenyl ephrine are the only oral OTC decongestants
available in the U.S. These oral decongestants are available as monotherapy
and in combination products. Cough and cold combination products frequently
contain an analgesic, antihistamine, cough modulator, and/or decongestant.
These combination products are often more convenient to the patient due to a
decreased pill burden and cost. However, similar to nonpregnant patients,
pregnant patients should use only the analgesic and cough and cold products
that address their symptoms. This will help minimize potential risks from the
use of unnecessary medications.
Pseudoephedrine and
phenylephrine are pregnancy category C in all three trimesters of pregnancy.
12 The American College of Obstetricians and Gynecologists (ACOG) and
the American College of Allergy, Asthma and Immunology (ACAAI) recommend using
pseudoephedrine during pregnancy. However, they advise against the use of oral
decongestants during the first trimester because of the potential increased
risk of gastroschisis (an abdominal wall defect).12 Retrospective
studies have shown an increased risk of gastroschisis with pseudoephedrine.
10,13,14 However, gastroschisis is a relatively rare condition, and a
higher risk does not guarantee that the adverse event will occur. One
prospective study of 453 women using decongestants in their first trimester
showed no elevated risk for malformations.14 Unfortunately, the
study population may not have been large enough to eliminate the risk of
gastroschisis.
Oral decongestants may also
result in vasoconstriction, which can induce maternal hypertension and lead to
impaired blood flow to the fetus. Since impaired blood flow can hinder fetal
growth, the risks of taking oral decongestants in the first trimester may
outweigh the benefits.
In the second and third
trimesters, pseudoephedrine can be recommended to pregnant patients in
appropriate doses. To minimize exposure to the fetus, pregnant patients should
take the immediate-release dosage form (instead of the extended-release) and
take the minimum effective dose for the shortest duration possible. An
appropriate dose is 30 to 60 mg every four to six hours as needed (maximum 240
mg/day).11
Oral decongestants are
vasoconstrictors and should not be used in patients with certain cardiac
diseases, such as uncontrolled hypertension and acute myocardial infarction.
They also have sympathomimetic properties and may aggravate some medical
conditions, such as diabetes mellitus and hyperthyroidism. The patient should
contact her physician if she has a high-risk pregnancy, a fever, or other
signs of infection, if the congestion lasts longer than seven days, or if the
medication does not relieve symptoms.11
Nasal:
Oxymetazoline, phenylephrine, naphazoline, and xylometazoline are commonly
available nasal decongestants in the U.S. All these nasal sprays/drops are
pregnancy category C. The amount of fetal exposure is minimal due to the small
amount of medication absorbed systemically. Few studies are available for any
of the nasal preparations. However, one prospective study of 197 and 56 women
exposed to intranasal oxymetazoline and phenylephrine, respectively, did not
show an increased risk for malformations.14
The American Pharmacists
Association's Handbook of Non-Prescription Drugs recommends using
oxymetazoline as the preferred nasal decongestant during pregnancy.11
Appropriate doses of oxymetazoline can be advised for patients during
pregnancy provided that the patient does not have any contraindications to the
drug. Contraindications include a high-risk pregnancy, fever or any other sign
of infection, and congestion longer than seven days. These products should be
used cautiously, if at all, in patients who cannot take oral decongestants.
The presence of underlying conditions (e.g., diabetes mellitus) and the level
of control of those disease states should be assessed before recommending the
nasal sprays or drops. An appropriate dose of oxymetazoline is two to three
sprays per nostril every 10 to 12 hours (maximum two doses per day). It is
important that patients be instructed not to use the medication more often
than recommended or longer than three days, due to the risk of rebound
congestion. If the medication is not effective, the patient should refer to
her physician.11
Expectorants and Antitussives
Guaifenesin:
Coughing is a protective reflex. Guaifenesin works to break up the mucus in
the patient's chest to make the cough more productive. If the patient is able
to cough up more of the mucus, the cough will likely decrease in frequency as
the mucus is cleared. However, guaifenesin has not been proven effective
against cough in patients with common cold symptoms.11,15,16
Appropriate alternative recommendations include a humidifier or vaporizer,
hydration, and hard candy.
Guaifenesin is considered
pregnancy category C. Guaifenesin has not been studied as extensively as other
OTC products. In one study of 197 pregnant women, there was an association
between guaifenesin exposure in the first trimester and an increased incidence
of inguinal hernias.17 This inguinal hernia association was not
found in other guaifenesin studies.6
Guaifenesin is
contraindicated in patients who have a chronic cough due to asthma, cigarette
smoking, emphysema, chronic bronchitis, heart failure, or
angiotensin-converting enzyme (ACE) inhibitor use. Fortunately, emphysema,
chronic bronchitis, and heart failure are relatively rare in women who are of
childbearing age. Furthermore, ACE inhibitor use is also traditionally avoided
in this patient subset. Other types of cough that should not be self-medicated
include coughs longer than seven days in duration, coughs that
decrease/disappear and return, and coughs in combination with symptoms of
infection, such as fever. Similar to other OTC cough and cold products, the
longer-acting, extended-release, and/or alcohol-containing preparations should
be avoided to minimize exposure to the fetus. An appropriate dose is 200 to
400 mg every four hours as needed (maximum 2,400 mg/day). See table 3 for
specific circumstances when patients should not be self-treated for a cough
and should be referred to a physician.
Dextromethorphan:
Since coughing may be protective, it should generally not be suppressed except
in certain situations. If the cough is not productive and interferes with
sleep, or it is severe in nature, it can be suppressed.
Similar to guaifenesin,
dextromethorphan has not been shown to be effective in patients with common
cold symptoms.11,16,18 Nonpharmacologic treatment similar to that
of guaifenesin can be recommended. Dextromethorphan is equipotent to codeine
as an antitussive and is a pregnancy category C medication. Dextromethorphan
exposure in the first trimester has been studied, and no increased risk of
malformations was detected.6 However, one study showed
teratogenicity when dextromethorphan was injected into avian embryos.19
Whether the data from avian embryos can be extrapolated to humans was
questioned and studied. In 128 women with a first-trimester exposure to
dextromethorphan, there were three major and seven minor malformations (versus
five major and eight minor malformations in the control group).20
This study demonstrated that the risk of malformations with dextromethorphan
was similar to the baseline rate of malformations. However, there is still
theoretical concern that an antagonist at the N-methyl-d-aspartate
receptor might affect fetal brain growth. To date, this adverse effect has not
been studied in humans.
Concurrent use of
dextromethorphan with central nervous system (CNS) depressants and monoamine
oxidase (MAO) inhibitors (within 14 days) should be avoided. It has the same
contraindications as guaifenesin therapy. An appropriate dose of
dextromethorphan is 10 to 20 mg every four hours as needed (maximum 120
mg/day).
In 2006, the American
College of Chest Physicians (ACCP) issued new guidelines addressing the
appropriate management of cough. Since the available OTC cough products do not
relieve the underlying cause, ACCP advises against the use of cough
suppressants and expectorants for cough due to postnasal drip. For the
postnasal drip cough, an antihistamine or decongestant is recommended. Given
that guaifenesin and dextromethorphan have questionable efficacy for cough
related to the common cold, they should be used sparingly at most in pregnant
patients. Nonpharmacologic measures for cough may prove more effective with
less risk to the patient.21
Antihistamines
Antihistamines
may decrease rhinorrhea and sneezing but do not affect other common cold
symptoms.11 The key OTC exception is loratadine, which does not
possess potent anticholinergic activity. Thus, loratadine does not treat
either rhinorrhea or sneezing from a nonallergic source. According to the
position statement issued by ACAAI and ACOG, chlorpheniramine was selected as
one of two recommended antihistamines in pregnancy (the other is not available
in the U.S.).12
Chlorpheniramine,
clemastine, diphenhydramine, and loratadine are considered pregnancy category
B. Brompheniramine and triprolidine are pregnancy category C. The most common
concerns about antihistamine use in pregnancy are cleft palate (loratadine and
diphenhydramine), polydactyly (diphenhydramine), retrolental fibroplasias, and
uterine contractions (diphenhydramine).22 A cause-and-effect
relationship for cleft palate and polydactyly could not be established due to
small sample sizes. An association was found between antihistamine use in the
last two weeks of pregnancy and an increased risk of retrolental fibroplasia.
23 When used in the third trimester, high-dose diphenhydramine may have
oxytocic properties. This may cause uterine contractions. Due to
lack of information and some theoretical risk, antihistamines should be
avoided in the late stages of pregnancy.
Several studies have
examined antihistamine use in the first trimester and have not shown an
increased risk of major malformations over those expected at baseline. Two
possible exceptions are brompheniramine and clemastine (limb reduction
defects). However, a cause-and-effect relationship has yet to be found.
Chlorphen iramine and diphenhydramine have not been associated with major
malformations in either the first trimester or at any time in pregnancy.
Triprolidine (plus pseudoephedrine) exposure in the first trimester has been
studied in 628 women.6 Of those studied, nine had a major
congenital abnormality. Whether this was caused by triprolidine or
pseudoephedrine could not be determined due to concurrent use.
Antihistamines should be
used with caution with CNS depressants, MAO inhibitors, and phenytoin. Caution
is also advised regarding antihistamine use if the patient has concurrent
narrow-angle glaucoma, peptic ulcer disease, asthma, emphysema, or chronic
bronchitis. Patients should be warned that they may have motor impairment even
if they do not feel drowsy. Other anticholinergic side effects are also
possible. Adult doses are as follows (as needed): 4 mg of brompheniramine
every four to six hours (maximum 24 mg/day), 4 mg of chlorpheniramine every
four to six hours (maximum 24 mg/day), 1.34 mg of clemastine every 12 hours
(maximum 2.68 mg/day), 2.5 mg of triproline every four to six hours (maximum
10 mg/day), and 25 to 50 mg of diphenhydramine every four to six hours
(maximum 300 mg/day)
Miscellaneous
Menthol and
Camphor: Menthol
and camphor have not been well studied in pregnancy. Menthol is a common
ingredient of many throat lozenges, sprays, and topical ointments. There are
no human studies on the use of menthol during pregnancy; thus, its risk is
undetermined. The concentration of menthol in these products is low, and the
risk of malformations is therefore believed to be small. Retrospective studies
with a camphor-based product (Vicks VapoRub) have not shown any developmental
toxicity associated with exposures during pregnancy.6 This product
should not be ingested orally. However, the American Pharmacist's
Association's Handbook on Non-Prescription Drugs recommends
patients consult their physician before using these medications.
Echinacea:
Echinacea is a common herbal medication used to stimulate the immune system.
The evidence available to support the use of echinacea for decreasing the
severity and duration of cold symptoms is controversial. The lack of
standardization in the product, differing preparations used, problems with
study design, and conflicting results make efficacy interpretation difficult.
24 One small study showed that the use of echinacea in the first
trimester did not increase the risk of major malformations. The results of the
study proved that echinacea was safe as short-term treatment (five to seven
days).25 Due to questionable efficacy and limited safety data,
echinacea should be avoided in pregnant women.
Zinc:
Zinc is commonly used to reduce the signs and symptoms of the common cold
when administered within 24 hours of symptom onset.26 Zinc lozenges
have been shown to be effective in reducing the duration of cold symptoms by a
modest amount.27 Trials involving zinc nasal sprays have not been
as promising.28,29
However, due to the
unpleasant taste of zinc lozenges, they are not easy to take. For the
treatment of cold symptoms, these lozenges, often unpalatable, must be
administered every two hours to be efficacious. The most common adverse effect
reported with zinc lozenges is nausea, which may be a preexisting problem in
this patient population.30 The zinc nasal gel may reduce the
likelihood of these side effects but lacks additional safety and efficacy data.
31
Only limited safety data are
available to support the use of zinc lozenges. However, several studies have
indicated that zinc supplementation in vitamins during pregnancy may improve
fetal development.32,33 Zinc has been proven safe in appropriate
doses during pregnancy. Doses for pregnant women older than 19 years should
not exceed 40 mg per day (34 mg/day for patients ages 14 to 18). Six drops per
day is recommended for some OTC zinc lozenges, which is equivalent to 79.9 mg
per day. If larger doses are taken, especially during the third trimester, the
patient's risk for complications, e.g., premature birth, is increased.
34 Pregnant women should be counseled on the importance of proper dosing
from all sources, including prenatal vitamins.
Vitamin C
Evidence
supporting the use of vitamin C to reduce the severity and duration of common
cold symptoms is debatable. In the studies that support vitamin C use for this
indication, the effects are modest (a decrease in symptoms by less than 24
hours). To achieve this outcome, the patient needs to take 1 to 3 g of vitamin
C per day. Doses larger than 1 g have been associated with an increase in side
effects, including nausea and diarrhea.35,36 Many pregnant patients
may find that the burden associated with high doses of vitamin C
administration may not be worth the potential benefit.
There is a limited amount of
safety data available to support vitamin C in pregnancy. However, at
appropriate doses, vitamin C appears to be safe during pregnancy.37
It is recommended that pregnant women older than 19 years do not take more
than 2 g of vitamin C per day (and less than 1,800 mg/day for pregnant
patients between ages 14 and 18).38 Practitioners and patients must
weigh the benefits against the risks when considering vitamin C during
pregnancy.
Role of the Pharmacist
Given that the
common cold is a self-limiting, non–life-threatening condition, and there is
some risk involved with any medication use in pregnancy, nonpharmacologic
treatment should be recommended before OTC medications. Hydration, rest,
vaporizers or humidifiers, nasal irrigation, and saline nasal sprays all help
symptom relief. Refer to tables 2 and 3 for conditions when the patient should
be referred to a physician and not self-medicated.
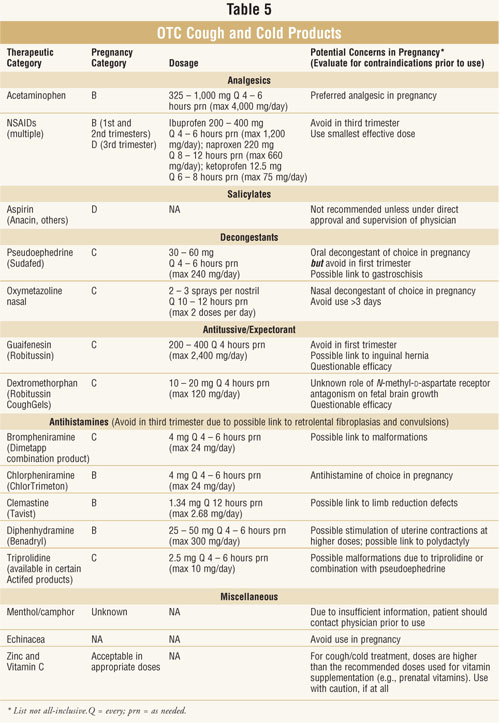
If a patient is an
appropriate candidate for self-treatment, see tables 4 and 5 for suitable
product choices. Pharmacists can help patients avoid combination therapy by
recommending medications that will directly address the symptoms that the
patient is experiencing. The pharmacist can advise the patient to avoid
products that may not work or that could be harmful. By cautioning the patient
against long-acting, alcohol-containing products, and encouraging dosage on an
as-needed basis, the pharmacist can help the patient minimize drug exposure to
the developing fetus. Thus, pharmacists have a vital role in guiding pregnant
women through the maze of OTC cough and cold products.
REFERENCES
1. Jacobs LR.
Prescription to over-the-counter drug reclassification. Am Fam Physician
. 1998;57:2209-2214.
2. Eisenberg DM,
Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United
States, 1990-1997: results of a follow-up national survey. JAMA.
1998;280:1569-1575.
3. Kaufman DW, Kelly
JP, Rosenberg L, et al. Recent patterns of medication use in the ambulatory
adult population of the United States: the Slone survey. JAMA.
2002;287:337–344.
4. Glover DD, Amonkar
M, Rybeck BF, Tracy TS. Prescription, over-the-counter, and herbal medicine
use in a rural, obstetric population. Am J Obstet Gynecol.
2003;188:1039-1045.
5. Meadows M.
Pregnancy and the drug dilemma. FDA Consum. 2001;35:16-20.
6. Briggs GG, Freeman
RK, Yaffe SJ. Drugs in Pregnancy and Lactation. 6th ed. Baltimore, Md:
Williams & Wilkins; 2002.
7. March of Dimes
Birth Defects Foundation. Available at:
www.marchofdimes.com/pnhec/4439_1206.asp. Accessed June 10, 2005.
8. Black RA, Hill DA.
Over-the-counter medications in pregnancy. Am Fam Physician.
2003;67:2517-2524.
9. Perinatology.com.
Index of exposures/drugs. Available at:
www.perinatology.com/exposures/druglists.htm. Accessed February 27, 2006.
10. Werler MM,
Sheehan JE, Mitchell AA. Maternal medication use and risks of gastroschisis
and small intestinal atresia. Am J Epidemiol. 2002;155:26-31.
11. Berardi RR,
McDermott JH, Newton GD. Handbook of Non-Prescription Drugs: An Interactive
Approach to Self-Care. Washington, DC: APhA Publications, 2004.
12. The use of newer
asthma and allergy medications during pregnancy. Position Statement. The
American College of Obstetricians and Gynecologists (ACOG) and the American
College of Allergy, Asthma and Immunology (ACAAI). Ann Allergy Asthma
Immunol. 2000;84:475-480.
13. Werler MM,
Mitchell AA, Shapiro S. First trimester maternal medication use in relation to
gastroschisis. Teratology. 1992;45:361-367.
14. Schatz M, Zeiger
RS, Harden K, et al. The safety of asthma and allergy medications during
pregnancy. J Allergy Clin Immunol. 1997;100:301-306.
15. Kuhn JJ, Hendley
JO, Adams KF, et al. Antitussive effect of guaifenesin in young adults with
natural colds: objective and subjective assessment. Chest.
1982;82:713-718.
16. Schroeder K,
Fahey T. Systematic review of randomised controlled trials of OTC cough
medicines for acute cough in adults. BMJ. 2002;324:329-331.
17. Heinonen OP,
Slone D, Shapiro S. Birth Defects and Drugs in Pregnancy. Littleton,
Mass: Publishing Sciences Group; 1977.
18. Lee PCL, Jawad
MSM, Eccles R. Antitussive efficacy of dextromethorphan in cough associated
with acute respiratory tract infections. J Pharm Pharmacol.
2000;52:1137-1142.
19. Andalaro V,
Monaghan D, Rosenquist T. Dextromethorphan and other N-methyl-d-aspartate
receptor antagonists are teratogenic in the avian embryo model. Pediatr Res
. 1998;43:1-7.
20. Einarson A,
Lyszkiewicz D, Koren G. The safety of dextromethorphan in pregnancy. Chest
. 2001;119:466-469.
21. Bolser DC. Cough
suppressant and pharmacologic protussive therapy: ACCP evidence-based
clinical practice guidelines. Chest. 2006;129:238S-249S.
22. Saxen I. Cleft
palate and maternal diphenhydramine intake. Lancet. 1974;1:407-408.
23. Zierler S,
Purohit D. Prenatal antihistamine exposure and retrolental fibroplasias. Am
J Epidemiol. 1986;123:192-196.
24. Giles JT, Palat
CT 3rd, Chien SH, et al. Evaluation of echinacea for treatment of the common
cold. Pharmacotherapy. 2000;20:690-697.
25. Gallo M, Sarkar
M, Au W, et al. Pregnancy outcome following gestational exposure to echinacea:
a prospective controlled study. Arch Intern Med. 2000;160:3141-3143.
26. Hulisz D.
Efficacy of zinc against common cold viruses: an overview. J Am Pharm Assoc
. 2004;44:594-603.
27. Prasad AS,
Fitzgerald JT, Bao B, et al. Duration of symptoms and plasma cytokine levels
in patients with the common cold treated with zinc acetate: a randomized,
double-blind, placebo-controlled trial. Ann Intern Med.
2000;133:245-252.
28. Turner RB.
Ineffectiveness of intranasal zinc gluconate for prevention of experimental
rhinovirus colds. Clin Infect Dis. 2001;33:1865-1870.
29. Belongia EA, Berg
R, Liu K. A randomized trial of zinc nasal spray for the treatment of upper
respiratory illness in adults. Am J Med. 2001;111:103-108.
30. Mossad SB,
Macknin ML, Medendorp SV, Mason P. Zinc gluconate lozenges for treating the
common cold: a randomized, double-blind, placebo-controlled study. Ann
Intern Med. 1996;125:81-88.
31. Hirt M, Nobel S,
Barron E. Zinc nasal gel for the treatment of common cold symptoms: a
double-blind, placebo-controlled trial. Ear Nose Throat J.
2000;79:778-782.
32. Merialdi M,
Caulfield LE, Zavaleta N, et al. Adding zinc to prenatal iron and folate
tablets improves fetal neurobehavioral development. Am J Obstet Gynecol
. 1999;180(2 pt 1):483-490.
33. Merialdi M,
Caulfield LE, Zavaleta N, et al. Randomized controlled trial of prenatal zinc
supplementation and the development of fetal heart rate. Am J Obstet Gynecol
. 2004;190:1106-1112.
34. DRI. Dietary
Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper,
Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. A
report of the Panel on Micronutrients and of Interpretation and Use of Dietary
Reference Intakes, and the Standing Committee on the Scientific Evaluation of
Dietary Reference Intakes Food and Nutrition Board, Institute of Medicine.
Washington, DC: National Academy Press; 2001.
35. Gorton HC, Jarvis
K. The effectiveness of vitamin C in preventing and relieving the symptoms of
virus-induced respiratory infections. J Manipulative Physiol Ther.
1999;22:530-533.
36. Douglas RM,
Chalker EB, Treacy B. Vitamin C for preventing and treating the common cold.
Cochrane Database Syst Rev. 2000;(2):CD000980.
37. Woods JR,
Plessinger MA, Miller RK. Vitamins C and E: missing links in preventing
preterm premature rupture of membranes? Am J Obstet Gynecol.
2001;185:5-10.
38. DRI: Dietary
Reference Intakes for Vitamin C, Vitamin E, Selenium and Carotenoids. A report
of the Panel on Dietary Antioxidants and Related Compounds, Subcommittees on
Upper Reference Levels of Nutrients and Interpretation and Uses of Dietary
Reference Intakes, and the Standing Committee on the Scientific Evaluation of
Dietary Reference Intakes. Food and Nutrition Board Institute of Medicine.
Washington, DC: National Academy Press; 2000.
To comment on this article,
contact editor@uspharmacist.com.





