US Pharm. 2023;48(8):33-37.
ABSTRACT: Measles is a highly communicable viral infection characterized by fever and rash. The virus can be transmitted to others by direct contact with infectious droplets or by airborne spread from 4 days before through 4 days after the onset of rash. Treatment is primarily supportive. Preventive measures include the use of live, measles-containing vaccines, isolation of suspected or confirmed cases, and the use of prophylaxis in select situations. Pharmacists are in a key position to advocate for vaccination, immunize eligible individuals as authorized by law, and work collaboratively with other healthcare professionals to ensure appropriate prevention and treatment of measles.
Measles illness is caused by the measles virus, an RNA paramyxovirus of the genus Morbillivirus.1 According to the CDC, measles was considered eliminated in the United States in 2000.2 However, in 2019, there were 1,274 cases of confirmed measles in 31 states, which represented the greatest number of cases in the U.S. since 1992.3 The majority of cases reported in this outbreak were in those who were not vaccinated.3 Since then, there were 13 cases reported in 2020, 49 cases in 2021, and 121 cases in 2022.3 Measles is more likely to occur in the U.S. in communities that have groups of unvaccinated individuals or in unvaccinated travelers to countries with an outbreak of measles.2-4
Transmission and Clinical Presentation
Measles is a highly communicable disease, with transmission rates of over 90% to those who are susceptible to infection and in close settings, such as members of the same household.5 Measles is transmitted from person to person via respiratory droplets, either through touching a surface that has droplets and then touching mucous membranes of the eyes, nose, or mouth, or through airborne transmission of aerosolized droplets.1,5,6 The measles virus can live for up to 2 hours in an airspace after the infected individual has left the space and can be spread from an infected individual from 4 days before the onset of rash through 4 days after the appearance of rash.1,5,6
The average duration between exposure to the virus and the development of symptoms is 7 to 14 days.7 The first symptoms of measles consist of a prodrome that lasts 3 to 5 days and is characterized by fever (as high as 103°F-105°F), cough, coryza, conjunctivitis (collectively referred to as the three Cs), and/or Koplik spots, which are blue-white spots that usually occur on the bright-red buccal mucosa located inside the cheeks.1,4,7 The characteristic maculopapular rash of measles follows the prodrome.1,7 The measles rash begins at the head and hairline, then advances to the face and upper neck and subsequently to the entire body, typically progressing in a pattern that is downward and outward and ending with the extremities of the hands and feet.1,7 The measles rash starts as flat red spots, and as the rash spreads, these spots may join together or develop small, raised bumps.7 The rash usually lasts 5 to 6 days and then fades in the order that it appeared on the body.1
Complications of measles infection are more common in children aged younger than 5 years, adults aged 20 years and older, and those who are immunocompromised.1,8 Diarrhea and otitis media are common complications of measles infection, whereas pneumonia and encephalitis are severe complications.8 Additionally, premature delivery and delivery of a low-birthweight baby are potential complications of measles in unvaccinated pregnant individuals.8 A rare, long-term complication of measles is a fatal, progressive degenerative neurological disorder called subacute sclerosing panencephalitis that may develop 7 to 10 years after recovery from the initial infection.4,8
Diagnostic Considerations
Measles should be suspected in individuals presenting with an illness characterized by rash and fever, especially in those with recent international travel or exposure to a person with similar symptoms.4 The most commonly used laboratory methods for confirmation of diagnosis are detection of measles-specific immunoglobulin M antibody from serum and polymerase chain reaction (PCR) detection of measles virus RNA from a respiratory specimen, typically collected from a swab of the throat or nasopharynx. PCR testing of urine samples to detect the measles virus and molecular testing to determine the genotype of the measles virus to help track pathways of transmission and assist with finding a source of the infection may also be performed.4
Management
Isolation
Individuals with measles should be isolated for 4 days after developing a rash to prevent transmission of infection to others.4 In the healthcare setting, it is important to rapidly identify and isolate all individuals with suspected or known measles.9 Healthcare personnel should adhere to standard and airborne precautions when managing patients with suspected or known measles, including immediate patient placement into an airborne infection isolation room and use of a respirator that is at least as protective as a fit-tested N95 respirator.9
Treatment
Currently, there are no FDA-approved agents for the treatment of measles.4 As such, supportive care, which consists of administering agents to provide symptomatic relief, is recommended.4 Measures to prevent the spread of infection to others, such as staying home for 4 days after the development of rash, washing hands, and disinfecting commonly touched surfaces, are also part of the management of measles.4,10 Additionally, the World Health Organization recommends vitamin A for children who are infected with measles to reduce the risk of complications, and this recommendation is also included in the CDC’s information on treatment.4,5
Prevention
Vaccines
Measles is a vaccine-preventable disease.5 Three live, attenuated vaccines are currently licensed by the FDA for the prevention of measles: two measles-mumps-rubella (MMR) vaccines, which are approved for use in those aged 12 months and older, and one measles-mumps-rubella-varicella (MMRV) vaccine under the trade name ProQuad (Merck), which has been available since 2005 and is approved for use in children aged 12 months through 12 years.11,12 The MMR vaccine under the trade name of M-M-R II (Merck) has been available in the U.S. since 1978, whereas the MMR vaccine under the trade name of PRIORIX (GlaxoSmithKline) has been available since 2022.13 According to the Advisory Committee on Immunization Practices (ACIP), M-M-R II and PRIORIX are considered fully interchangeable, even for off-label uses, and either vaccine may be used to prevent measles, mumps, and rubella when an MMR vaccine is indicated.13 The ACIP states that the rationale for the recommendation is that “two interchangeable vaccines from different manufacturers will help safeguard vaccine supply.”13 It is important to note that PRIORIX does not contain gelatin, which is in M-M-R II; however, PRIORIX contains latex in the tip caps of the prefilled syringes of diluent.13-15 Both M-M-R II and ProQuad can be given IM or SC, whereas PRIORIX should be administered SC.12,16 Additional information about measles-containing vaccines, including considerations for their use, is included TABLE 1.
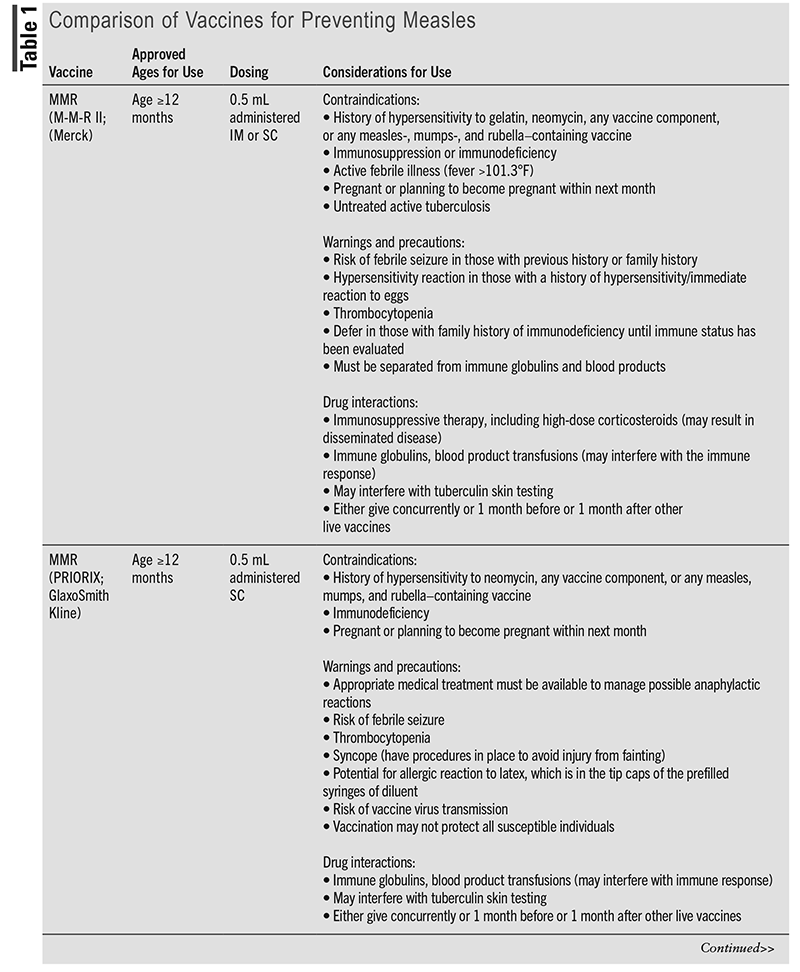
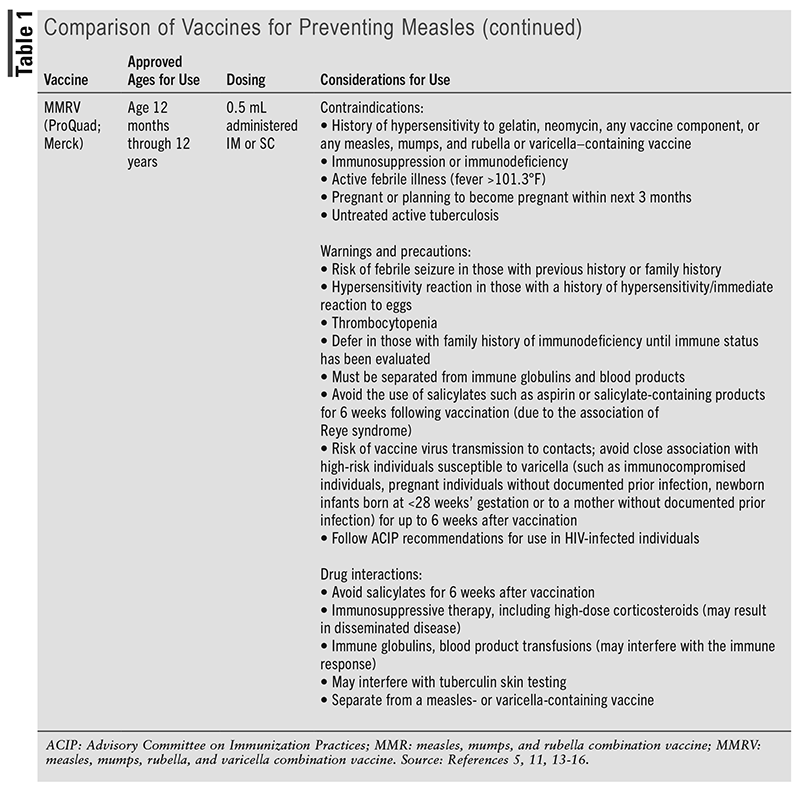
Recommendations for measles vaccines are summarized in TABLE 2, and the clinician should take into account the considerations for use of each vaccine, as indicated in TABLE 1. Routine vaccination with two doses of a measles-containing vaccine (either MMR or MMRV) is recommended in children, with the first dose at age 12 to 15 months, followed by the second dose at age 4 to 6 years prior to entry into school.11,17 Although MMR vaccines are approved for use in children aged 12 months and older, the ACIP recommends an MMR vaccine prior to international travel in infants aged between 6 and 12 months.11,13,17 However, a dose given to children aged younger than 12 months does not count as part of the routine childhood vaccine series. Therefore, children who receive a dose of vaccine prior to their first birthday should still receive two more doses (starting at age 12 months through 15 months) according to the recommended schedule.11,17
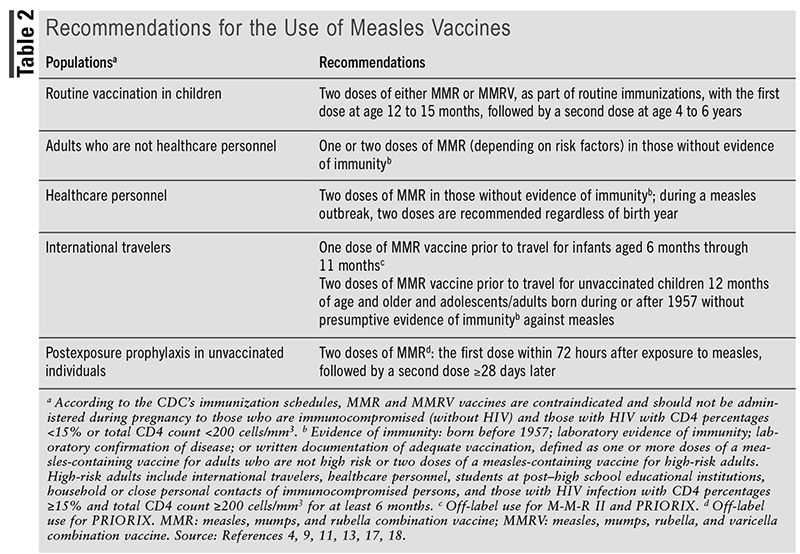
For adults without evidence of immunity, at least one dose of MMR vaccine is recommended for the prevention of measles.4,11,18 However, two doses should be given to adults in a setting that is considered to be high-risk, which includes international travelers, healthcare personnel, students at post–high school educational institutions, household or close personal contacts of immunocompromised persons, and those with HIV infection with CD4 percentages ≥15% and CD4 count ≥200 cells/mm3 for at least 6 months (since the MMR vaccine is contraindicated in those with HIV with CD4 percentage <15% or CD4 count <200 cells/mm3).4,11,18 It is also recommended to consider giving two doses of MMR vaccine to healthcare personnel born prior to 1957 who do not have laboratory evidence of immunity or laboratory confirmation of disease.18
Postexposure Prophylaxis
Individuals who are exposed to measles and do not have presumptive evidence of immunity should be offered postexposure prophylaxis with either MMR or immunoglobulin.4 MMR vaccine should be administered within 72 hours after initial exposure to measles, whereas immunoglobulin should be administered within 6 days of exposure to measles.4
Monitoring
Individuals infected with measles should be monitored for the development of complications of infection.4 Healthcare personnel who have been exposed to measles should be monitored daily for signs and symptoms of infection for 21 days after last exposure.9
Pharmacist’s Role
Pharmacists play an important role in the management and prevention of measles. Pharmacists are in a key position to educate the public and other healthcare professionals about measures to prevent the spread of infection and have an integral role in immunizations.
Conclusion
Measles is a viral infection characterized by fever and rash that is primarily managed with supportive treatment. Because measles is highly communicable to those who are susceptible to infection, preventive measures are key to controlling the spread of infection. Preventive measures include isolation of suspected or confirmed cases and the use of live, measles-containing vaccines. Pharmacists are well positioned to educate the public on measles vaccines, advocate for vaccination, and immunize eligible individuals as authorized by law. Pharmacists should work collaboratively with other healthcare professionals to ensure appropriate prevention and management of this highly transmissible infection.
REFERENCES
1. CDC. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hall E, Wodi AP, Hamborsky J, et al, eds. 14th ed. Washington, D.C.: Public Health Foundation, 2021. www.cdc.gov/vaccines/pubs/pinkbook/index.html. Accessed May 20, 2023.
2. Patel M, Lee AD, Clemmons NS, et al. National update on measles cases and outbreaks–United States, January 1–October 1, 2019. MMWR Morb Mortal Wkly Rep. 2019;68:893-896.
3. CDC. Measles (rubeola): measles cases and outbreaks. www.cdc.gov/measles/cases-outbreaks.html. Accessed May 20, 2023.
4. CDC. Measles (rubeola): for healthcare providers. www.cdc.gov/measles/hcp/index.html. Accessed May 20, 2023.
5. CDC Yellow Book 2024: Health Information for International Travel. New York: Oxford University Press; 2023.
6. CDC. Measles (rubeola): transmission of measles. www.cdc.gov/measles/transmission.html. Accessed May 20, 2023.
7. CDC. Measles (rubeola): signs and symptoms. www.cdc.gov/measles/symptoms/signs-symptoms.html. Accessed May 20, 2023.
8. CDC. Measles (rubeola): complications of measles. www.cdc.gov/measles/symptoms/complications.html. Accessed May 20, 2023.
9. CDC. Interim infection prevention and control recommendations for measles in healthcare settings, updated July 2019. www.cdc.gov/infectioncontrol/guidelines/measles/index.html. Accessed May 20, 2023.
10. CDC. Measles (rubeola): questions about measles. www.cdc.gov/measles/about/faqs.html. Accessed May 20, 2023.
11. CDC. Vaccines and preventable diseases: measles, mumps, and rubella (MMR) vaccination: what everyone should know. www.cdc.gov/vaccines/vpd/mmr/public/index.html#what-is-mmr. Accessed May 20, 2023.
12. Merck. US FDA approves intramuscular administration for Merck’s MMRV family of vaccines: M-M-RII (measles, mumps, and rubella virus vaccine live), VARIVAX (varicella virus vaccine live), and ProQuad (measles, mumps, rubella and varicella virus vaccine live). www.merck.com/news/us-fda-approves-intramuscular-administration-for-mercks-mmrv-family-of-vaccines-m-m-rii-measles-mumps-and-rubella-virus-vaccine-live-varivax-varicella-virus-vaccine-live-and-proquad/. Accessed May 20, 2023.
13. Krow-Lucal E, Marin M, Shepersky L, et al. Measles, mumps, rubella vaccine (PRIORIX): recommendations of the Advisory Committee on Immunization Practices–United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1465-1470.
14. M-M-R II (Measles, Mumps, and Rubella Virus Vaccine Live) suspension for intramuscular or subcutaneous injection package insert. Rahway, NJ: Merck & Co, Inc.; 2023.
15. PRIORIX (Measles, Mumps, and Rubella Vaccine, Live), suspension for subcutaneous injection package insert. Research Triangle Park, NC; GlaxoSmithKline; 2022.
16. ProQuad (Measles, Mumps, Rubella and Varicella Virus Vaccine Live) suspension for intramuscular or subcutaneous injection package insert. Rahway, NJ: Merck & Co, Inc.; 2023.
17. CDC. Recommended child and adolescent immunization schedule for ages 18 years or younger, United States, 2023. www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf. Accessed May 20, 2023.
18. CDC. Recommended adult immunization schedule for ages 19 years or older, United States, 2023. www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed May 20, 2023.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





