US Pharm. 2023;48(6):25-30.
ABSTRACT: Febrile neutropenia (FN), a potential complication of myelosuppressive chemotherapies for various solid and hematologic malignancies, can contribute to subsequent dose reductions or treatment delays. For chemotherapy regimens that carry a >20% risk of FN, prophylactic use of a granulocyte colony-stimulating factor (G-CSF) may be indicated. Several FDA-approved biosimilars of short-acting and long-acting G-CSFs are available. Antimicrobial prophylaxis is used in neutropenic patients in an attempt to prevent FN, with each regimen tailored per patient and disease-specific characteristics. In patients who develop high-risk FN after chemotherapy, front-line antibiotic options should provide broad-spectrum coverage, including pseudomonal infection. Pharmacists in outpatient and inpatient settings have numerous drug-related intervention opportunities for the prevention and treatment of FN in adults.
Neutrophils are part of the body’s first-line defense against microbial infections.1 These cells are polymorphonuclear leukocytes within the innate immune system, and their predominant functions are phagocytosis, degranulation, and formation of neutrophil extracellular traps.1
The 2010 Infectious Diseases Society of America (IDSA) guideline defines neutropenia as an absolute neutrophil count (ANC) <500 neutrophils/mcL (or an anticipated decline to ANC <500 over the next 48 hours) and profound neutropenia as ANC <100.2 The 2018 joint guideline of the American Society of Clinical Oncology and IDSA describes neutropenia as ANC <1,000, severe neutropenia as ANC <500, and profound neutropenia as ANC <100.3 The most recent National Comprehensive Cancer Network (NCCN) guideline defines neutropenia as ANC <500 (or <1,000 with a predicted decline to <500 over the next 48 hours).4 Fever is defined as either a single oral body temperature of >38.3°C (101°F) or >38.0°C (100.4°F) sustained over 1 hour.2-4 Febrile neutropenia (FN) is the concurrence of fever and low ANC as described above.2-5
The most recent Common Terminology Criteria for Adverse Events (CTCAE) classifies neutropenia as grade 1 (ANC 1,500), grade 2 (ANC 1,000-<1,500), grade 3 (ANC 500-<1,000), or grade 4 (ANC <500).6 The CTCAE categorizes FN as grade 3 (ANC <1,000 with fever) or grade 4 (life-threatening consequences; urgent intervention indicated).6
FN is a dose-limiting toxicity of chemotherapy regimens, and patients may require prolonged hospitalization with broad-spectrum antibiotics.4,5 Chemotherapy regimens have significant variations in respective rates of therapy-related neutropenia and FN, and either event may warrant future dose reductions and/or treatment delays. Known risk factors that impact FN outcomes include disease state (solid or hematologic malignancy), chemotherapy dosing (standard or high-dose), and organ dysfunction (renal or hepatic).4,5
Particularly during chemotherapy order-verification and dispensing phases, pharmacists play a large role in ensuring that doses and frequencies are optimized for a patient’s age, renal or hepatic function, CBC with differential (including WBC count, hemoglobin, and platelets), performance status, and/or prior tolerance of adverse effects (AEs). Pharmacists can also recommend appropriate prophylactic medications, if indicated.
Prevention
One strategy for mitigating infection during neutropenia is the addition of antimicrobial prophylaxis, which depends on the overall infection risk of the regimen and/or disease state.4 Per recent NCCN guidelines, no antimicrobial prophylaxis is indicated for low risk (anticipated neutropenia for <7 days; e.g., solid tumors) unless a patient had a prior herpes simplex virus (HSV) infection or reactivation. For intermediate risk (anticipated neutropenia for 7-10 days; e.g., autologous stem-cell transplant, lymphoma, multiple myeloma, chronic lymphocytic leukemia, purine analogue therapy) and high risk (anticipated neutropenia for >10 days; e.g., allogeneic stem-cell transplant, acute leukemia, alemtuzumab therapy, moderate-severe graft-versus-host disease), the NCCN guidelines include recommendations for bacterial, fungal, and viral prophylaxis. Bacterial prophylaxis, if used, includes administration of an antipseudomonal fluoroquinolone during neutropenia. Fungal prophylaxis should be considered during neutropenia or anticipated mucositis. Viral prophylaxis should be considered during treatment, periods of neutropenia, and potentially afterward, depending on the ongoing risk of HSV infection or reactivation.4 Although the NCCN recommendations are generalized, there will be significant variations among disease states, chemotherapy regimens, institution-specific protocols, and patient-specific factors.
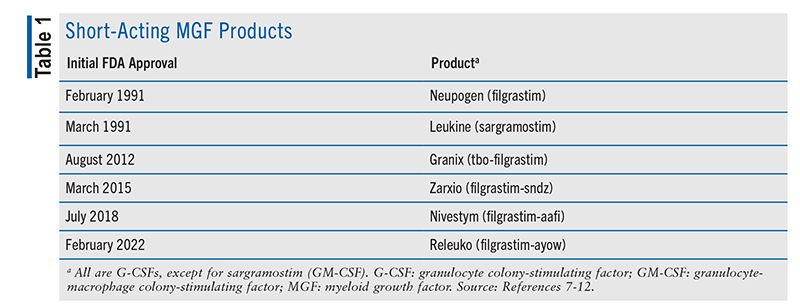
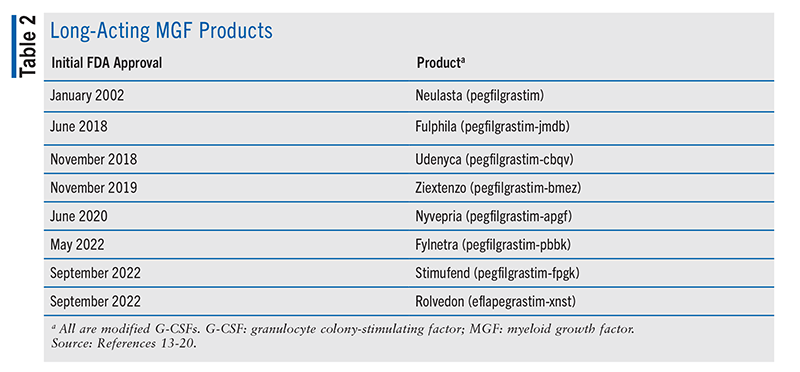
Another neutropenia-mitigation strategy is the use of a myeloid growth factor (MGF).4,5 For chemotherapy regimens with a high FN rate (>20%), prophylactic use of an MGF, such as granulocyte colony-stimulating factor (G-CSF), is strongly recommended.4,5 TABLE 1 and TABLE 2 summarize the G-CSF products currently approved by the FDA.7-20 See FIGURE 1 for the schematic structures of G-CSF products. For regimens with an intermediate FN rate (10%-20%), prophylactic G-CSF is an important consideration in patients who have one or more independent risk factors (prior chemotherapy, prior radiation, persistent neutropenia, bone-marrow involvement by malignancy, recent surgery or open wounds, renal or hepatic dysfunction, full-dose chemotherapy in those aged >65 years).4,5 For regimens with a low FN rate (<10%), prophylactic G-CSF is not routinely recommended but may be considered based on the aforementioned patient risk factors or prior chemotherapy intolerability.4,5
If a patient develops FN after a chemotherapy cycle despite prophylactic G-CSF, the chemotherapy dosing or regimen should be reevaluated.4,5 If a patient develops FN after a cycle of chemotherapy without prophylactic G-CSF, incorporation of prophylactic G-CSF should be considered for subsequent cycles.4,5
Short-Acting MGF Products
Current FDA-approved G-CSF drugs (TABLE 1) include two unique biological products (filgrastim, tbo-filgrastim) and three biosimilars of filgrastim (-sndz, -aafi, -ayow).7-12,21 As described in their package inserts (PIs), each of these products is a recombinant nonglycosylated G-CSF with an N-terminal methionine developed via Escherichia coli.7-12 These drugs contain a sequence of 175 amino acids, and their molecular weight (MW) is approximately 18.8 kDa. With the exception of filgrastim-sndz (available only as single-dose prefilled syringes), each filgrastim-related product is available as single-dose vials (300 mcg/mL, 480 mcg/1.6 mL) and single-dose prefilled syringes (300 mcg/0.5 mL, 480 mcg/0.8 mL).7-12 Latex-free products include tbo-filgrastim and filgrastim-aafi.9,11
The registration trial (i.e., study leading to FDA approval) for filgrastim evaluated its safety and efficacy for reducing the incidence of infection or FN after myelosuppressive chemotherapy in nonmyeloid malignancies.7,22 This randomized‚ double-blind‚ placebo-controlled trial included solid-tumor (small-cell lung cancer) patients who received up to six cycles of IV chemotherapy with cyclophosphamide/doxorubicin on day 1 and etoposide on days 1 through 3 of a 21-day cycle. Patients were randomized to SC filgrastim 230 mcg/m2 (~4-8 mcg/kg/day; n = 99) or placebo (n = 111) starting on day 4 and continuing for a maximum of 14 days. In total, 210 patients were evaluated for efficacy and 207 for safety. Baseline demographics and disease characteristics were similar between groups; patients had a median age of 62 years (range, 31-80 years), 64% were male, 89% were Caucasian, 72% had extensive disease, and 28% had limited disease. The primary efficacy endpoint was FN incidence, which was 40% for filgrastim-treated patients and 76% for placebo patients (P <.001). Filgrastim significantly reduced the incidence and overall duration of infection related to FN; incidence, severity, and duration of severe neutropenia (ANC <500); incidence and overall duration of hospital admission; and antibiotic duration. The most common AEs were fever, pain, rash, cough, and dyspnea.7,22 PIs for biosimilar drugs also reference these study data.8-12
The registration trial for tbo-filgrastim evaluated its safety and efficacy in 348 chemotherapy-naïve solid-tumor (high-risk stage 2-4 breast cancer) patients who were receiving doxorubicin 60 mg/m2 and docetaxel 75 mg/m2.9,23 This multinational, multicenter, randomized, controlled, phase III study compared tbo-filgrastim with placebo and a filgrastim product serving as a control. Dosing for each agent was 5 mcg/kg SC once daily, given 1 day after completion of chemotherapy for >5 days (until postnadir ANC >10,000 or maximum 14 days). Patients’ median age was 50 years (range, 25-75 years) at baseline, and 86% of patients were Caucasian. For the outcome of duration of severe neutropenia, tbo-filgrastim was superior to placebo (1.1 days vs. 3.8 days, P <.0001).9,23 The most common AE was bone pain.9,23
The only FDA-approved granulocyte-macrophage CSF is sargramostim (Leukine); however, this agent is not currently indicated for preventing chemotherapy-induced FN after myelosuppressive chemotherapy in nonmyeloid malignancies.8
Long-Acting MGF Products
Drugs currently approved by the FDA (TABLE 2) include two unique biological products (pegfilgrastim, eflapegrastim-xnst) and six biosimilars of pegfilgrastim (-jmdb, -cbqv, -bmez, -apgf, -pbbk, -fpgk).13-20 As detailed in the PIs for pegfilgrastim-related products, each drug is a covalent conjugate of recombinant methionyl human G-CSF (filgrastim) and monomethoxypolyethylene glycol.13-19 The MW of pegfilgrastim is about 39 kDa. Pegfilgrastim and its biosimilars are available as single-dose prefilled syringes (6 mg/0.6 mL) for SC injection.13-19 Pegfilgrastim (originator product only) is also available with a copackaged on-body injector that delivers the drug approximately 27 hours after skin application.13 Eflapegrastim-xnst is produced via covalent coupling of a human G-CSF analogue and an Fc fragment of human immunoglobulin G4, both of which are derived from recombinant E coli, via a single polyethylene glycol linker.20 The recombinant G-CSF domain in eflapegrastim-xnst is a variant of human G-CSF with two serine substitutions at positions 17 and 65 and no additional N-terminal methionine. Eflapegrastim-xnst has an MW of about 72 kDa. Eflapegrastim-xnst is available as a single-dose prefilled syringe (13.2 mg/0.6 mL) for SC injection.20 Latex-free long-acting MGF products include specific pegfilgrastim biosimilars (-jmdb, -cbqv, -apgf, -pbbk) and eflapegrastim-xnst.14,15,17,18,20
The registration trials for pegfilgrastim were randomized, double-blind, and controlled.13,24-26 Study 1 (Green et al; fixed-dose pegfilgrastim) and study 2 (Holmes et al; weight-adjusted pegfilgrastim) used doxorubicin 60 mg/m2 and docetaxel 75 mg/m2 administered every 21 days for up to four cycles in patients with solid tumors (metastatic breast cancer).13,24,25 In study 1, 157 patients were given pegfilgrastim 6 mg for one dose or filgrastim 5 mcg/kg/day beginning on day 2 of each chemotherapy cycle.13,24 In study 2, 310 patients received pegfilgrastim 100 mcg/kg or filgrastim 5 mcg/kg/day beginning on day 2 of each chemotherapy cycle.13,25 In both studies, the mean number of days of severe neutropenia after chemotherapy cycle 1 did not differ significantly between pegfilgrastim and filgrastim groups.24,25 Study 3 (Vogel et al) used docetaxel 100 mg/m2 administered every 21 days for up to four cycles in patients with solid tumors (metastatic or nonmetastatic breast cancer).13,26 In this study, 928 patients received pegfilgrastim 6 mg or placebo for one dose on day 2 of each chemotherapy cycle. The pegfilgrastim group had a lower incidence of FN compared with placebo-treated patients (1% vs. 17%; P <.001). Incidences of hospitalizations (1% vs. 14%) and IV anti-infective use (2% vs. 10%) were also lower in the pegfilgrastim group.13,26 The most common AEs were bone pain and extremity pain.13,24-26
The two registration trials (study 1 and study 2) for eflapegrastim-xnst evaluated the drug’s efficacy in decreasing the incidence of infection (due to FN) in patients with nonmyeloid malignancies who received myelosuppressive chemotherapy.20,27,28 These 1:1 randomized, open-label, active-controlled, similarly designed, noninferiority studies included a total of 643 patients with solid tumors (early-stage breast cancer).20,27,28 Docetaxel 75 mg/m2 and cyclophosphamide 600 mg/m2 were administered every 21 days for up to four cycles. A fixed dose of eflapegrastim-xnst (13.2 mg/0.6 mL) or pegfilgrastim (6 mg/0.6 mL) was administered on day 2 of each cycle after chemotherapy. At baseline, patients’ median age was 60 years (range, 24-88 years); 77% of patients were Caucasian, and 12% were African American.20,27,28 Study 1 (Schwartzberg et al) assessed 406 patients receiving either eflapegrastim-xnst (n = 196) or pegfilgrastim (n = 210).20,27 Study 2 (Cobb et al) included 237 patients receiving either eflapegrastim-xnst (n = 118) or pegfilgrastim (n = 119).20,28 In both trials, the duration of severe neutropenia after chemotherapy cycle 1 was not significantly different between the eflapegrastim-xnst and pegfilgrastim groups.20,27,28 The most common AEs from eflapegrastim-xnst were pain (bone, muscle, joint, back), headache, fever, fatigue, nausea, diarrhea, anemia, and rash.20,27,28
According to NCCN guidelines, FDA-approved biosimilars are appropriate substitutes for filgrastim (Neupogen) and pegfilgrastim (Neulasta) originators.5 Pharmacists can participate in formulary management of these products and work with oncology teams to ensure that preferred biosimilar product pathways are developed or implemented. If a third-party payer (i.e., insurance company) does not cover a particular short-acting or long-acting G-CSF product, the pharmacist can help the provider select an appropriate substitute. If a patient has a documented latex allergy, the pharmacist can advise about alternative products that are latex free.
Treatment
The NCCN guidelines state that if a patient has FN, the medical history (e.g., major comorbidities, type or time of prior chemotherapy, prior infections, recent antibiotic use, relevant environmental exposures, CBC with differential, comprehensive metabolic panel, chest x-ray, urinalysis) should be assessed.4 Additionally, a microbiological evaluation (e.g., blood or urine cultures, site-specific or localizing symptom diagnostics) should be performed.4
The NCCN considers patients to be at low risk for complications of FN if they lack high-risk factors; had outpatient status when fever developed; have no acute comorbidities warranting inpatient treatment; have anticipated short duration of severe neutropenia (ANC <100 for <7 days); have good performance status (European Cooperative Oncology Group 0-1); have no renal or hepatic impairment; or have a Multinational Association of Supportive Care in Cancer (MASCC) Risk Index score >21 or Clinical Index of Stable Febrile Neutropenia (CISNE) score <3.4 Critical laboratory values should be reviewed, and the appropriateness of oral antibiotic therapy should be assessed. Patients should also be evaluated for social criteria: adequate home environment, 24-hour home caregiver, access to a telephone, access to emergency facilities, and distance within 1 hour of a medical center or treating physician’s office. Outpatient treatment options include at-home IV antibiotics, at-home or clinic-administered daily long-acting IV agent with or without oral therapy, and at-home oral therapy only (e.g., ciprofloxacin plus amoxicillin/clavulanate, levofloxacin). Patients should be monitored daily (e.g., home or clinic visit for first 72 hours, then telephone follow-up), and they should return to clinic if they have positive test cultures, new signs or symptoms, persistent or recurrent fever after 3 to 5 days, or inability to tolerate prescribed antibiotics. An alternative (but less preferable) approach to outpatient management is observation for 2 to 12 hours to confirm low-risk status, ensure clinical stability, administer first antibiotic dose and monitor for reactions, and provide education, with telephone follow-up within 12 to 24 hours.4
The NCCN considers patients to be at high risk for complications of FN if they had inpatient status when fever developed; have significant comorbidities or clinical instability; had allogeneic stem-cell transplantation; have anticipated prolonged duration of severe neutropenia (ANC <100 for >7 days); have renal (creatinine clearance <30 mL/min) or hepatic impairment (aspartate transaminase/alanine transaminase >5 times upper limit of normal); have uncontrolled or progressive malignancy; have pneumonia or complex infection; have grade 3-4 mucositis; or have an MASCC Risk Index score <21 or CISNE score >3.4 Initial antibiotic therapy should be based on consideration of infection-risk assessment, broad-spectrum coverage with antipseudomonal activity, colonization or prior multidrug-resistant organisms, infection site, local antibiotic-susceptibility patterns (antibiograms), organ dysfunction, allergy history, and prior antibiotic history. IV antibiotic monotherapy options include cefepime, piperacillin/tazobactam, ceftazidime, meropenem, and imipenem/cilastatin. Site- or symptom-specific evaluation of mouth/mucosal membranes, esophagus, and sinus/nasal areas should be performed, and any abdominal or perirectal pain, diarrhea, vesicular lesions, and urinary tract, lung, skin, vascular-access device, or central nervous system signs and symptoms should be investigated. Therapy may be modified to cover additional organisms (e.g., fluconazole for oral thrush; vancomycin for suspected methicillin-resistant Staphylococcus aureus).4 Infectious-disease consultation is generally recommended to determine the most appropriate therapy and duration, and it should be more highly considered in patients with a history of recurrent or resistant infections.
Hypothetical Case
Narrative: RS is a 55-year-old female with refractory multiple myeloma. Her body weight is 86 kg, and she has no renal or hepatic impairment. RS is given inpatient VD-PACE (bortezomib, dexamethasone, cisplatin, doxorubicin, cyclophosphamide, etoposide) therapy and filgrastim 5 mcg/kg/day starting on day 6 (>24 hours after completing chemotherapy). On day 9, her ANC is 50, body temperature is 39°C, blood pressure is 90/60 mmHg, and heart rate is 110 bpm; she has chills and bone pain, with no other site-specific symptoms. RS has a documented history of anaphylaxis with penicillin. After an expedited evaluation (including urinalysis, chest x-ray, and cultures), the primary team starts aztreonam 2 g IV every 8 hours, vancomycin 15 mg/kg IV every 12 hours, acetaminophen 650 mg for one dose, Lactated Ringer’s solution 2 L bolus for one dose, and loratadine 10 mg daily. RS’s hemodynamics improve, but she still has profound neutropenia (ANC <100). The team decides to consult the infectious-disease service the following day to determine the optimal treatment duration.
Discussion: This case describes high-risk FN; the patient’s MASCC Risk Index score is <21.29 RS received standard doses of chemotherapy and did not require any adjustment for organ dysfunction. This regimen has a known risk (>20%) of FN; therefore, filgrastim was included in the protocol.5,30 Cefepime monotherapy would be indicated, but the choice of antibiotics was modified based on RS’s allergy history.2 If RS were to continue vancomycin beyond 48 hours, therapeutic drug monitoring (e.g., goal trough levels 15-20 mcg/mL) would be warranted.4 Infectious-disease consultation would be prudent in this case to determine optimal antimicrobial therapy and duration. RS received acetaminophen for fever, as well as Lactated Ringer’s solution for initial hypotension. Loratadine was started as an adjunct treatment for filgrastim-related bone pain.31
Conclusion
FN is a known complication of several chemotherapy regimens, and it is associated with significant morbidity and mortality. It is prudent for the medical team to take precautions against FN. Pharmacists (both inpatient and outpatient) can have a significant impact on evaluation of the appropriateness of required G-CSF support, antimicrobial prophylaxis, and/or potential need for chemotherapy dose adjustments. Although most patients will have high-risk FN, there is a selected patient population that may qualify for outpatient therapy. Regardless of setting, pharmacists play a key role in assessing the appropriateness of antimicrobial therapy and providing the medical team with recommendations on dosing and/or potential alternative agents that may be required. The roles of the pharmacist in FN are critical to the optimization of patient and healthcare outcomes. It is essential that pharmacists in all practice settings be aware of the prophylactic and therapeutic management of FN.
REFERENCES
1. Rosales C. Neutrophil: a cell with many roles in inflammation or several cell types? Front Physiol. 2018;9:113.
2. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Executive summary: clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the IDSA. Clin Infect Dis. 2011;52(4):427-431.
3. Taplitz RA, Kennedy EB, Bow EJ, et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America clinical practice guideline update. J Clin Oncol. 2018;36(14):1443-1453.
4. National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Prevention and treatment of cancer-related infections. Version 3.2022. www.nccn.org/professionals/physician_gls/pdf/infections.pdf. Accessed January 27, 2023.
5. NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Hematopoietic growth factors. Version 1.2023. www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf. Accessed January 27, 2023.
6. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Accessed January 27, 2023.
7. Neupogen (filgrastim) product information. Thousand Oaks, CA: Amgen Inc; February 2021.
8. Leukine (sargramostim) product information. Lexington, MA: Partner Therapeutics Inc; May 2022.
9. Granix (tbo-filgrastim) product information. North Wales, PA: Teva Pharmaceuticals USA, Inc; November 2019.
10. Zarxio (filgrastim-sndz) product information. Princeton, NJ: Sandoz Inc; August 2022.
11. Nivestym (filgrastim-aafi) product information. Lake Forest, IL: Hospira, Inc. November 2021.
12. Releuko (filgrastim-ayow) product information. Bridgewater, NJ: Amneal Pharmaceuticals LLC; April 2022.
13. Neulasta (pegfilgrastim) product information. Thousand Oaks, CA: Amgen Inc; February 2021.
14. Fulphila (pegfilgrastim-jmdb) product information. Morgantown, WV: Mylan Pharmaceuticals, Inc; October 2021.
15. Udenyca (pegfilgrastim-cbqv) product information. Redwood City, CA: Coherus BioSciences, Inc; November 2022.
16. Ziextenzo (pegfilgrastim-bmez) product information. Princeton, NJ: Sandoz Inc; March 2021.
17. Nyvepria (pegfilgrastim-apgf) product information. Lake Forest, IL: Hospira, Inc; October 2021.
18. Fylnetra (pegfilgrastim-pbbk) product information. Bridgewater, NJ: Amneal Pharmaceuticals LLC; May 2022.
19. Stimufend (pegfilgrastim-fpgk) product information. Lake Zurich, IL: Fresenius Kabi USA, LLC; September 2022.
20. Rolvedon (eflapegrastim-xnst) product information. Irvine, CA: Spectrum Pharmaceuticals, Inc; September 2022.
21. FDA. Purple Book database of licensed biological products. https://purplebooksearch.fda.gov. Accessed January 27, 2023.
22. Crawford J, Ozer H, Stoller R, et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991;325(3):164-170.
23. del Giglio A, Eniu A, Ganea-Motan D, et al. XM02 is superior to placebo and equivalent to Neupogen™ in reducing the duration of severe neutropenia and the incidence of febrile neutropenia in cycle 1 in breast cancer patients receiving docetaxel/doxorubicin chemotherapy. BMC Cancer. 2008;8:332.
24. Green MD, Koelbl H, Baselga J, et al. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol. 2003;14(1):29-35.
25. Holmes FA, O’Shaughnessy JA, Vukelja S, et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol. 2002;20(3):727-731.
26. Vogel CL, Wojtukiewicz MZ, Carroll RR, et al. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol. 2005;23(6):1178-1184.
27. Schwartzberg LS, Bhat G, Peguero J, et al. Eflapegrastim, a long-acting granulocyte-colony stimulating factor for the management of chemotherapy-induced neutropenia: results of a phase III trial. Oncologist. 2020;25(8):e1233-e1241.
28. Cobb PW, Moon YW, Mezei K, et al. A comparison of eflapegrastim to pegfilgrastim in the management of chemotherapy-induced neutropenia in patients with early-stage breast cancer undergoing cytotoxic chemotherapy (RECOVER): a phase 3 study. Cancer Med. 2020;9(17):6234-6243.
29. Medscape. MASCC febrile neutropenia risk. https://reference.medscape.com/calculator/134/mascc-febrile-neutropenia-risk. Accessed January 27, 2023.
30. Abdallah AO, Mohyuddin GR, Ahmed N, et al. Outcomes of VDPACE with an immunomodulatory agent as a salvage therapy in relapsed/refractory multiple myeloma with extramedullary disease. EJHaem. 2021;2(4):757-764.
31. Kirshner JJ, McDonald MC III, Kruter F, et al. NOLAN: a randomized, phase 2 study to estimate the effect of prophylactic naproxen or loratadine vs no prophylactic treatment on bone pain in patients with early-stage breast cancer receiving chemotherapy and pegfilgrastim. Support Care Cancer. 2018;26(4):1323-1334.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





