ABSTRACT: Continuous subcutaneous insulin infusion (CSII) devices, commonly referred to as insulin pumps, are a growing trend in diabetes care. Insulin pumps enable patients to program basal delivery of insulin per hour, as well as deliver boluses for food intake throughout the day. Benefits include better diabetes control, increased flexibility of basal delivery, precise bolus dosing, and improved quality of life. Limitations may include cost, insurance coverage, and fear of dependence; CSII has also been associated with infusion-site reactions, diabetic ketoacidosis, and retinopathy. A variety of pumps with features such as alarms, reminders, and Bluetooth connectivity are available. Pharmacists can play an important role in providing pump education and training for patients, as well as troubleshooting and pump management.
US Pharm. 2017;42(11)Epub.
The first insulin pump was developed by Dr. Arnold Kadish in 1963.1,2 Since then, significant strides in pump design have been made, with improvements in safety, ease of use, and efficacy. It is currently estimated that one million individuals worldwide use continuous subcutaneous insulin infusion (CSII) therapy to manage their diabetes mellitus (DM); most are patients with type 1 diabetes (T1DM), but those with type 2 diabetes may also use CSII therapy.3,4 In the United States, as of 2007, approximately 375,000 adults with T1DM used external infusion pumps.5
The Diabetes Control and Complications Trial (DCCT) demonstrated the importance of intensive insulin therapy to delay the onset and slow the progression of diabetic microvascular complications, such as retinopathy, nephropathy, and neuropathy.6 A study completed in 2015 also found benefits for patients with macrovascular complications. Over 18,000 T1DM patients on CSII versus multiple daily injections (MDI) therapy were evaluated, and the authors found that CSII was associated with a 45% reduction in fatal coronary heart disease, a 42% reduction in fatal cardiovascular disease, and a 27% reduction in all-cause mortality.7
Although CSII therapy is a viable option for intensive insulin therapy, it is vital that potential pump users are screened to determine their appropriateness as CSII candidates. Candidates for CSII therapy include those with hemoglobin A1C (HbA1c) values above goal, frequent hypoglycemia, and/or large fluctuations in blood glucose (BG) levels. The ideal CSII candidate is also highly motivated, has access to educational and support resources, and is comfortable with pump technology; however, many patients may be successful with pumps regardless of their initial comfort level. Patients also must be willing to test their BG at least four times daily to reduce the risk of hyperglycemic crises, such as diabetic ketoacidosis or hyperosmolar hyperglycemic syndromes, and to determine changes in pump settings that may be needed to get their BG to goal.3,8,9
Although these suggested criteria are useful in determining who might succeed in attaining glycemic goals on pump therapy, the clinician’s judgment is critical in selecting appropriate candidates for initiating use of an insulin pump. Once a potential candidate is identified, the patient must weigh the advantages and drawbacks of CSII therapy and make an informed decision based on personal treatment options. There are numerous resources available for patients and healthcare providers (see Sidebar 1). This article will provide an overview of the benefits and limitations of CSII therapy, as well as discuss features of, and differences in the insulin pumps that are currently available in the U.S.
Basic Mechanics Of Insulin Pumps
Insulin pumps are computerized devices that are capable of delivering insulin in extremely small, accurate increments. The patient is able to program the device for basal delivery of insulin per hour, as well as to deliver boluses for food intake throughout the day, based on the individual’s insulin-to-carbohydrate (IC) ratios and insulin sensitivity factors (ISFs). The IC ratio refers to the grams of carbohydrate covered by one unit of insulin, and the ratio is used to calculate the number of units-to-bolus for meals and snacks. An ISF is the decrease in BG, measured in milligrams/deciliter, caused by one unit of insulin; ISFs are helpful in determining correction doses when patients have high BG.3
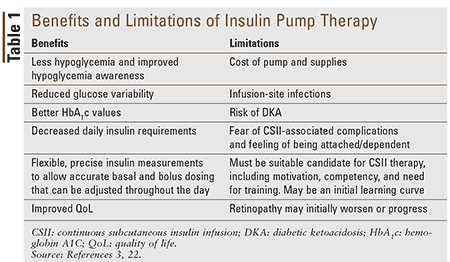
MDI therapy typically consists of an injection of intermediate or long-acting insulin and three injections of rapid or short-acting insulin for meals.3 Although still commonly used, long-acting insulin must be given in fixed doses, with little adaptability to changes in daily routines. CSII therapy uses rapid-acting insulin—insulin aspart (Novolog), insulin glulisine (Apidra), and insulin lispro (Humalog)—which allows for frequent and adjustable basal rates to be administered from the pump. Delivery of insulin is often done with the use of infusion sets. Infusion sets are small plastic tubes that connect to a plastic cannula or needle that is placed subcutaneously. The infusion set is connected to a reservoir that stores insulin. The goal of CSII is to mimic the pancreas’ physiologic release of insulin in the body.3 Table 1 summarizes the benefits and limitations of CSII therapy.
Benefits of Pump Therapy
Better DM Control: There is substantial evidence demonstrating better patient outcomes with CSII therapy compared with MDI. These improved outcomes include less frequent hypoglycemia, increased hypoglycemia awareness, lower HbA1c values, and reduced glucose variability.10-17 A meta-analysis by Pickup and Sutton found a statistically significant reduction in severe hypoglycemia in both the pediatric and adult populations, with a 2.9-fold decrease in events.10 Reductions in HbA1c vary among studies and have been seen in adult, pediatric, and adolescent patients.11,15,16 Evidence has shown that the greatest HbA1c benefit will occur with initiation of CSII in a patient with a higher baseline HbA1c value. If the patient’s baseline HbA1c was 10%, CSII resulted in an average of 0.65% lower HbA1c values versus MDI therapy. However, if the baseline HbA1c was 6.5%, there was no HbA1c reduction benefit of CSII versus MDI therapy.11 A 4-month trial found an average HbA1c lowering of 0.35% in patients utilizing CSII therapy versus MDI (P <.001),15 whereas another study found an average decrease in HbA1c of 0.9% after 3 months of CSII therapy (P <.03) and an average decrease of 0.7% after 6 months (P <.05).16 There is evidence that smaller daily amounts of insulin are required to manage DM with CSII compared with MDI.18-20 A randomized, controlled trial by Hoogma and colleagues found that pump therapy reduced daily insulin amounts by 26% compared with MDI (P <.0001).18 Overall, there are many benefits to CSII therapy when it comes to improving DM control.
Increased Flexibility of Basal Delivery: Basal rates may be altered throughout the day to allow for varying insulin needs posed by meals, stress, exercise, and daily lifestyle variations. This flexibility can help overcome problems such as dawn phenomenon—an unusually high BG in the early morning hours—because the patient is able to decrease basal rates during the early morning hours. This increased fasting BG is due to the release of growth hormones, such as cortisol, that cause increased hepatic glucose production and insulin sensitivity. BG can be controlled by increasing the overnight supply of insulin or by increasing basal rates of insulin in patients on CSII therapy.3,21
Insulin doses may be given in increments as small as 0.001 units. This precision in insulin dosing can be especially beneficial to patients who are sensitive to insulin or those who require only small amounts of insulin per day.3 All insulin pumps have a temporary basal setting that allows for a lower or higher portion of the basal dose to be delivered for a certain time period, a benefit for individuals with spontaneous changes in basal need (e.g., exercise, sick days, etc.).3,22
Precise Bolus Dosing: Every pump contains a bolus calculator that recommends, on the screen, a bolus amount to be given, depending on the individual’s programmed IC ratio, ISF, target BG, and insulin-on-board—the amount of insulin remaining from recent boluses that may still be lowering BG. Patients must enter their current BG and the grams of carbohydrates they will consume. After the pump recommends a bolus dose, the patient must take the initiative to administer the bolus. Currently, there is no pump that self-delivers insulin doses. This is a common misconception that patients may have about CSII therapy. If patients are not counting carbohydrates or monitoring BG before each meal (typically not recommended), they are able to deliver manual boluses that can be determined prior to each bolus or set as a “preset bolus” for frequently used amounts to make the bolus process simpler. Patients should be educated on the bolus dosing features of insulin pumps before starting therapy.3
Improved Quality of Life: Patients on CSII describe an improved quality of life (QoL) compared with MDI. Much research has been done that supports these results. Patients report fewer injections, less hypoglycemia, more freedom and flexibility with meals and day-to-day variations, easier problem solving, and greater convenience than MDI.18,23-25 Hoogma and colleagues administered a diabetes-specific QoL questionnaire to patients and noted a higher score association with CSII therapy (P <.001).18 Another study utilized the DCCT questionnaire and described patient-reported improvements in daily convenience with CSII therapy.23 There is also evidence of improvements in family cohesion in patients on CSII rather than MDI.26 Patients are also able to discreetly wear their insulin pump underneath their clothing. Based upon self-reported questionnaires on diabetes treatment satisfaction and fear of hypoglycemia, T1DM patients experienced statistically significantly higher satisfaction rates and reduced hypoglycemia fear with CSII therapy compared with MDI.12
Limitations of Pump Therapy
Cost: Price can be an obstacle for many patients. Insurance may cover most of the expenses, but there will be out-of-pocket costs that the patient will be responsible for paying. It is important to know what these will be before committing to starting CSII therapy.3 To enable the patient to understand up-front and ongoing costs, an investigation of the patient’s benefits should be completed. Some insurance carriers may require additional laboratory tests to demonstrate that the patient is truly insulin dependent. These tests could include C-peptide, fasting BG, and insulin autoantibody levels. Depending on insurance coverage, the expenses of a pump and pump supplies may be more costly than those for MDI.22 However, research suggests that CSII therapy may be more cost-effective than MDI in the long term, particularly because of improved DM control and fewer DM complications.27,28
Infusion-Site Reactions: CSII is associated with increased risk of infusion-site reactions compared with MDI, including contact dermatitis and infections; these are the most common complications associated with pump therapy.29-31 This increased risk is due to the infusion set of a pump being in place for 2 to 3 days at a time. Use of sterile techniques may help prevent skin infections; this includes washing hands thoroughly with soap and water prior to inserting an infusion set; avoiding breathing on the infusion set or site and avoiding touching the face during this process; using an alcohol pad to cleanse the skin of bacteria before insertion; and changing the infusion set frequently (at a minimum, every 72 hours).3,31
Diabetic Ketoacidosis: It is thought that with the use of rapid-acting insulin only, the failure of the pump to deliver insulin to a patient with T1DM could lead to low levels of insulin in the body after only a few hours. The absence of insulin results in the body’s inability to utilize glucose for fuel, resulting in lipolysis and the hepatic metabolism of free fatty acids from adipocytes. This process results in ketone bodies, an acidic byproduct, which can lead to an acidic state called diabetic ketoacidosis (DKA).32 Complications of DKA include dehydration, electrolyte disturbances, cerebral edema, cognitive impairment, venous thrombosis, aspiration, cardiac arrhythmias, pancreatic-enzyme elevations, and death.33 The use of long-acting insulin with MDI therapy makes DKA less likely to occur. Despite this theory, research has not demonstrated an increased risk of DKA for CSII compared with MDI.6,29,34 However, DKA is a potentially serious complication for DM patients, and CSII therapy should always be closely monitored for appropriate insulin delivery to prevent DKA. Patients starting on CSII therapy should be educated on the importance of recognizing signs and symptoms of DKA, how to monitor for ketones using urine and blood ketone strips, and how to treat if DKA is suspected.3
Fear of Feeling Dependent on a Pump: While on CSII therapy, patients are rarely without their insulin pump, in contrast to patients using MDI, who are able to inject their insulin and remove themselves from their medication. Because of this, patients may feel apprehensive about being attached to or dependent on the insulin pump, especially children and adolescents.25,35 However, as stated previously, after getting comfortable with and accustomed to using the pump, patients may experience an increase in QoL owing to the flexibility it allows and level of BG control gained.18,23-25
Retinopathy: In the DCCT, patients with moderate or advanced retinopathy experienced worsened symptoms with intensive insulin therapy compared with conventional therapy within the first year of CSII treatment. This worsening of symptoms often disappeared within 18 months.6 It is thought that rapid improvements in BG may result in increased vascular endothelial growth factor (VEGF) levels, which can lead to proliferative retinopathy and macular edema.36-39 To prevent this from occurring, it is recommended that BG control be slowly improved over months to prevent the release of VEGF.3 Follow-up with an ophthalmologist is also vital to prevent eye damage and retinopathy when CSII is initiated. It is recommended that patients with HbA1c values greater than 9% receive dilated retinal exams every 3 to 6 months during the first year of CSII therapy.22
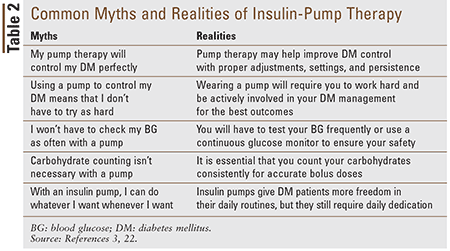
Table 2 discusses many misconceptions about CSII therapy. It is important for patients to meet with a certified insulin-pump trainer or certified diabetes educator to discuss their concerns before starting on an insulin pump.
Available Pumps and Features
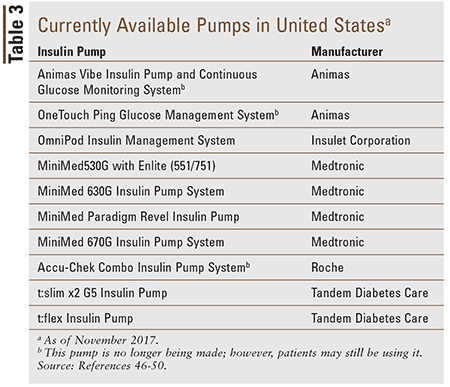
Table 3 lists the insulin pumps currently available in the U.S. by prescription. Medtronic has developed the first hybrid closed-loop pump, the MiniMed 670G System, which has the ability to self-adjust insulin doses based on the patient’s current BG; it also allows programmable thresholds when a continuous glucose monitor is worn in addition to the pump. It has been FDA approved and is now available for patient use.40
Associated-Meter or Continuous Glucose Monitor: Most insulin pumps on the market are associated with a glucometer or continuous glucose monitor (CGM). Communication between the meter or CGM and the insulin pump allows the BG level to be sent directly to the insulin pump using Bluetooth or radio-frequency technology. This eliminates the need for the patient to input the measured BG into his or her bolus calculator. This automation has many benefits, including speeding up the process of calculating a bolus dose, increasing accuracy, and decreasing risk of incorrect bolus doses given because of an incorrect BG entry. The integration also allows for a more comprehensive history that can be combined into one database to improve the confidence of providers in recommending adjustments to basal rates, bolus doses, IC ratios, and ISFs to optimize patient outcomes.3,22
It is currently recommended that CGMs be used in the management of patients with DM.41,42 A CGM is a sensor that is inserted subcutaneously to monitor BG and the trend of the patient’s BG. The patient wears the CGM for 6 or 7 days before discarding it and replacing it with a new sensor. Some of the current insulin pumps are integrated with a CGM and can receive BG data from the sensor. If the CGM is not connected to a pump, the sensor can wirelessly transmit BG to a handheld receiver. The FDA recently approved the use of the Dexcom G5 CGM to adjust insulin doses.43 The use of CGMs is associated with a greater reduction in HbA1c compared with self-monitoring BG (SMBG).44,45 A recent trial by Lind and colleagues found a mean reduction of 0.43% in HbA1c with CGM compared with conventional treatment with MDI and SMBG (P <.001).44 CGM has also been shown to decrease the risk of hypoglycemia.44,45 The DIAMOND Randomized Clinical Trial found that patients in the CGM arm experienced hypoglycemia significantly less than patients in the control arm did (P = .002).45
Tubeless Pumps: Currently, the only wearable pump that does not require tubing to connect the pump to the infusion site is the Omnipod (Insulet). This pump adheres to the skin directly. The Omnipod uses a wireless personal data manager that works in conjunction with the pump to insert a flexible plastic cannula directly from the wearable device and administer boluses, and it serves as a glucometer. Instead of the infusion set and tubing being replaced, as would be required with traditional insulin pumps, the entire Omnipod is removed and discarded every 2 to 3 days (depending on insulin requirement).46
Alarms and Reminders: Alarms and reminders vary among individual pumps. However, there are many types of reminders that a patient can use. These can include low battery, low cartridge (selected number of units left in reservoir), delivery limit, reminder to test BG and change infusion site, high or low glucose alert, automatic sleep, alert for a missed meal, and alarms for other special features the pump may possess. Newer CGM technology also allows alarms to be sent to mobile devices of the patients’ friends or family members in the event of BG going above or below programmed thresholds. Prediction alerts utilizing BG trend data have the capability to warn of a predicted high or low BG in advance of reaching these thresholds. These settings may be adjusted based on the patient’s preferences.3
Additional Pump Features: With upgrades in technology, many pumps have multicolor screens. Some pumps have touch screens, similar to smartphones. Bluetooth and other wireless-technology compatibility allows patients to download their pump, glucometer, or CGM data directly to smartphone applications or computers. These downloaded data may be shared with patients’ healthcare professionals, allowing for easily accessible information and trend data for the healthcare team. Pumps may have food libraries stored within them that provide information on carbohydrate content. Additional accessories, such as remote controls, are also available.3,46-50
Pharmacist Involvement
Pharmacists can be involved in the care of patients receiving CSII therapy by becoming a certified diabetes educator or a certified pump trainer for one or more insulin-pump companies. These certifications may require additional training and education, a specified number of hours of experience with DM patients, and successful completion of competency examinations.3,51 There are community and ambulatory-care pharmacists in the U.S. who have successfully implemented CSII initial training, management, and education of patients with DM. These individuals have been able to receive reimbursement for their services.51
Conclusion
Many diabetic patients have opted to control their DM with CSII therapy, and the number of insulin-pump patients is expected to increase in the coming years.3 DM management with CSII can provide many benefits to patients. However, it also poses some risks that should not be taken lightly. Patients considering CSII therapy should learn about the benefits and limitations of insulin pumps so that they can make an informed decision. When deciding upon a specific pump, CSII candidates should research all available pumps, compare and contrast each one, and choose the pump that they feel will assist in improving their particular difficulties in managing DM successfully.
REFERENCES
1. Tamborlane WV, Sherwin RS, Genel M, Felig P. Reduction to normal of plasma glucose in juvenile diabetes by subcutaneous administration of insulin with a portable infusion pump. N Engl J Med. 1979;300(11):573-578.
2. Pickup JC, White MC, Keen H, et al. Long-term continuous subcutaneous insulin infusion in diabetics at home. Lancet. 1979;8148:870-873.
3. Walsh J, Roberts R. Pumping Insulin: Everything You Need to Succeed on an Insulin Pump. 5th ed. San Diego, CA: Torrey Pines; 2012. 1-92; 103-136; 181-188.
4. American Diabetes Association. Insulin pumps need greater safety review: American Diabetes Association issues joint statement with European Association for the Study of Diabetes. March 16, 2015. www.diabetes.org/newsroom/press-releases/2015/insulin-pumps.html. Accessed November 30, 2016.
5. FDA. Insulin infusion pumps panel information. General Hospital and Personal Use Medical Devices Panel. March 2010.
6. The Diabetes Control and Complication Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
7. Steineck I, Cederholm J, Eliasson B, et al. Insulin pump therapy, multiple daily injections, and cardiovascular mortality in 18,168 people with type 1 diabetes: observational study. BMJ. 2015;350:h3234.
8. American Association of Diabetes Educators 2008 Consensus Summit. Insulin pump therapy: guidelines for successful outcomes. Chicago, IL: September 18, 2008. 2-10.
9. American Association of Diabetes Educators. Education for continuous subcutaneous insulin infusion pump users. Diabetes Educ. 2003;29:97-99.
10. Pickup JC, Sutton AJ. Severe hypoglycemia and glycaemic control in Type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med. 2008;25:765-774.
11. Retnakaran R, Hochman J, DeVries JF, et al. Continuous subcutaneous insulin infusion versus multiple daily injections. The impact of baseline A1c. Diabetes Care. 2004;27:2590-2596.
12. Little SA, Leelarathna L, Walkinshaw E, et al. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2x2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care. 2014;37:2114-2122.
13. Willi SM, Planton J, Egede L, Schwarz S. Benefits of continuous subcutaneous insulin infusion in children with type 1 diabetes. J Pediatr. 2003;143:796-801.
14. Linkeschova R, Raoul M, Bott U, et al. Less severe hypoglycaemia, better metabolic control, and improved quality of life in Type 1 diabetes mellitus with continuous subcutaneous insulin infusion (CSII) therapy: an observational study of 100 consecutive patients followed for a mean of 2 years. Diabet Med. 2002;19:746-751.
15. Hanaire-Broutin H, Melki V, Bessieres-Lacombe S, Tauber JP. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens using insulin lispro in type 1 diabetic patients on intensified treatment: a randomized study. The Study Group for the Development of Pump Therapy in Diabetes. Diabetes Care. 2000;23:1232-1235.
16. Sulli N, Shashaj B. Continuous subcutaneous insulin infusion in children and adolescents with diabetes mellitus: decreased HbA1c with low risk of hypoglycemia. J Pedtr Endocrinol Metab. 2003;16:393-399.
17. Weintrob N, Schechter A, Benzaquen H, et al. Glycemic patterns detected by continuous subcutaneous glucose sensing in children and adolescents with type 1 diabetes mellitus treated by multiple daily injections vs continuous subcutaneous insulin infusion. Arch Pediatr Adolesc Med. 2004;158:677-684.
18. Hoogma RP, Hammond PJ, Gomis R, et al. Comparison of the effects of continuous subcutaneous insulin infusion (CSII) and NPH-based multiple daily insulin injections (MDI) on glycaemic control and quality of life: results of the 5-nations trial. Diabet Med. 2006;23:141-147.
19. Coloquitt JL, Green C, Sidhu MK, et al. Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes. Health Technol Assess. 2004;8(43):1-186.
20. Pickup J, Mattock M, Kerry S. Glycaemic control with continuous subcutaneous insulin infusion compared with intensive insulin injections in patients with type 1 diabetes: meta-analysis of randomised controlled trials. BMJ. 2002;324(7339):705.
21. Koivisto V, Yki-Jarvinen H, Helve E, et al. Pathogenesis and prevention of the dawn phenomenon in diabetic patients treated with CSII. Diabetes. 1986;(1):78-82.
22. Potti L, Haines S. Continuous subcutaneous insulin infusion therapy: a primer on insulin pumps. Pharmacy Today. 2009;15(1):54-71.
23. Chantelau E, Schiffers T, Schutze J, Hansen B. Effect of patient-selected intensive insulin therapy on quality of life. Patient Educ Couns. 1997;30:167-173.
24. Hirsch IB, Bode BW, Garg S, et al. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care. 2005;28:533-538.
25. Scheidegger U, Allemann S, Scheidegger K, Diem P. Continuous subcutaneous insulin infusion therapy: effects on quality of life. Swiss Med Wkly. 2007;137:476-482.
26. Shapiro J, Wigg D, Charles MA, Perley M. Personality and family profiles of chronic insulin-dependent diabetic patients using portable insulin infusion pump therapy: a preliminary investigation. Diabetes Care. 1984;7(2):137-142.
27. Roze S, Valentine WJ, Zakrzewska KE, Palmer AJ. Health-economic comparison of continuous subcutaneous insulin infusion with multiple daily injection for the treatment of Type 1 diabetes in the UK. Diabet Med. 2005;22(9):1239-1245.
28. Cohen N, Minshall ME, Sharon-Nash L, et al. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin: economic comparison in adult and adolescent type 1 diabetes mellitus in Australia. Pharmacoeconomics. 2007;25(17):881-897.
29. Lenhard MJ, Reeves GD. Continuous subcutaneous insulin infusion: a comprehensive review of insulin pump therapy. Arch Intern Med. 2001;161:2293-3000.
30. Mecklenburg RS, Benson EA, Benson JW Jr, et al. Acute complications associated with insulin pump therapy: report of experience with 161 patients. JAMA. 1984;252:3265-3269.
31. Pietri A, Raskin P. Cutaneous complications of chronic continuous subcutaneous insulin infusion therapy. Diabetes Care. 1981;4:624-626.
32. Castillo MJ, Scheen AJ, Lefebvre PJ. The degree/rapidity of the metabolic deterioration following interruption of a continuous subcutaneous insulin infusion is influenced by the prevailing blood glucose level. J Clin Endocrinol Metab. 1996;81(5):1975-1978.
33. Hirsch I, Emmett M. Diabetic ketoacidosis and hyperosmolar hyperglycemic state in adults: clinical features, evaluation, and diagnosis. UpToDate. www.uptodate.com/contents/diabetic-ketoacidosis-and-hyperosmolar-hyperglycemic-state-in-adults-clinical-features-evaluation-and-diagnosis. Accessed January 23, 2017.
34. Bending JJ, Pickup JC. Complications of insulin infusion pump therapy. JAMA. 1985;253:2644-2645.
35. Tamborlane WV, Swan K, Sikes KA, et al. The renaissance of insulin pump treatment in childhood type 1 diabetes. Rev Endocr Metab Disord. 2006;7:205-213.
36. Brinchmann-Hansen O, Dahl-Jorgensen K, Hanssen KF, Sandvik L. The response of diabetic retinopathy to 41 months of multiple insulin injections, insulin pumps, and conventional insulin therapy. Arch Ophthalmol. 1988;106(9):1242-1246.
37. Van Ballegooie E, Hooymans JM, Timmerman Z, et al. Rapid deterioration of diabetic retinopathy during treatment with continuous subcutaneous insulin infusion. Diabetes Care. 1984;7:236-242.
38. Dahl-Jorgensen K, Brinchmann-Hansen O, Hanssen KF, et al. Rapid tightening of blood glucose control leads to transient deterioration of retinopathy in insulin dependent diabetes mellitus: the Oslo study. Br Med J (Clin Res Ed). 1985;290(6471):811-815.
39. Jeganathan VS. Anti-angiogenesis drugs in diabetic retinopathy. Curr Pharm Biotechnol. 2011;12(3):369-372.
40. Medtronic. MiniMed 670G system highlights. 2017. www.medtronicdiabetes.com/products/minimed-670g-insulin-pump-system. Accessed February 3, 2017.
41. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(Suppl1) S48-S55.
42. American Association of Clinical Endocrinologists (AACE). AACE/ACE consensus conference on continuous glucose monitoring. Washington, DC. February 20, 2016. http://media.aace.com/sites/aace.newshq.businesswire.com/files/press_kit/file/CGM_summary_integrated_comments.FINAL_.pdf. Accessed January 19, 2017.
43. FDA. FDA News Release. FDA expands indication for continuous glucose monitoring system, first to replace fingerstick testing for diabetes treatment decisions. December 20, 2016. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm534056.htm. Accessed February 3, 2017.
44. Lind M, Polonsky W, Hirsch I, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317(4):379-387.
45. Beck R, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317(4):371-378.
46. Omnipod system. Product comparison. How Omnipod stacks up.
https://myomnipod.com/explore-Omnipod/product-comparison/. Accessed September 21, 2017.
47. Animas. Our pumps. The Animas insulin pumps & CGM system. August 9, 2016. www.animas.com/diabetes-insulin-pump-and-blood-glucose-meter. Accessed January 19, 2017.48. MiniMed Insulin Pump Therapy. Medtronic. 2017. www.medtronicdiabetes.com/home. Accessed January 19, 2017.
49. Accu-Chek. Accu-Chek combo system. www.accu-chek.com/insulin-pumps-integrated-systems/combo-system/support. Accessed January 19, 2017.
50. Tandem Diabetes Care. Products. 2017. www.tandemdiabetes.com/products. Accessed January 19, 2017.
51. Boyd LC, Boyd ST. Insulin pump therapy training and management: an opportunity for community pharmacists. J Manag Care Pharm. 2008;14(8):790-794.
To comment on this article, contact rdavidson@uspharmacist.com.