US Pharm. 2010;35(10):13-21.
The switching of prescription products to nonprescription status has been one of the most dynamic movements in self-care.1-3 These Rx-to-OTC switches cause profound changes in the medical arena.4 Patients are able to self-treat with a new medication, and, occasionally, the condition is one that has never before been amenable to self-treatment. Pharmacist responsibilities increase as patients bring questions about the new product.
Questions the Pharmacist Should Consider
There are several questions the pharmacist should attempt to answer as a switch becomes imminent1:
• Is there a significant therapeutic advantage to this product for each of its advertised indications? Answering this question will involve examining the package labeling of all products in the same category. If the indication is one that was not previously self-treatable, the product is “first-in-class,” presenting a significant therapeutic gain in self-care. This was the case with alli, Plan B, Rogaine, and Nicorette.1 If, on the other hand, the newly switched product’s FDA-approved labeling reveals few significant differences when compared to another product currently on the market, there may be no compelling reason to recommend it.
• Does the labeling on the outside of the package reflect all of the important information patients should know regarding product use, or is some information hidden inside? Some switched products have booklets inside the package that cover important counseling information (e.g., alli). The concerned pharmacist will wish to open one package to examine this material in advance to be fully prepared to counsel on switched products.
• Was the product switch preceded by a “launch kit” that provided all of the important information needed to counsel on the product? Responsible manufacturers send these advance tool kits to pharmacies prior to introduction of the new product. The launch kit will probably contain window signs, buttons, Mylar balloons, and other flashy advertising materials, but ideally the professionally oriented launch kit should also delineate and explain all drug interactions, precautions in use, contraindications, age limitations, dosing regimens, and any other important counseling information. All such labeling is confidential prior to marketing, as it will have been negotiated between the FDA and the manufacturer. If the manufacturer refuses to inform the pharmacist about this vital information via a launch kit or other avenue prior to the date the product hits store shelves, the pharmacist is forced to undertake a crash course in the labeling when the product first arrives, just at the time that demand is at its highest point due to publicity.
• For price-conscious patients, is the product’s price significantly lower or higher than other products in the same category?
Questions to Ask Patients
When a patient inquires about a newly switched product, several general questions should be asked:
• Did a physician (or other prescriber) recommend that you use this product? If the patient answers in the affirmative, the pharmacist seldom needs to go any further.
• What condition do you wish to treat? The pharmacist must check to ensure that the patient’s condition is actually one of the labeled indications for the product.
• Have you ever been medically diagnosed with the condition of concern, or do you just think you have it? Publicity surrounding a newly switched product may lead some consumers into guessing they might have the condition. The pharmacist can help them determine whether the problems they are experiencing actually match those normally caused by the condition in question.
• Have you ever been given a prescription for this medication? If the patient answers in the affirmative, the pharmacist should investigate further to see whether the patient should continue to see the physician (e.g., the patient with an active ulcer). If the patient has a recent prescription but the physician has denied refills, the patient should be urged to return to the physician rather than self-treat.
If the pharmacist determines that the answers to the general questions may allow self-use, more specific questions about the age of the patient, duration of the condition, and patient contraindications should follow.
Recent Switches
Prevacid 24HR: When a product is switched, one of the most critical questions is the potential impact to the patient. In 2003, Prilosec OTC (omeprazole) was the first proton pump inhibitor (PPI) to be switched to OTC status.5-7 Since that time, it has become a significant force in the heartburn/reflux market, competing successfully against antacids and histamine-2 (H2) antagonists. Six years later, Prevacid 24HR (lansoprazole) became the second PPI available without a prescription.5 Prilosec’s 6-year market exclusivity allowed it to establish a firm place in the nonprescription arena. Ideally, Prev-acid 24HR would have possessed one or more compelling therapeutic advantages (as reflected in the FDA-approved labeling) that could be leveraged in its marketing campaign. However, a close examination of the labels of Prilosec OTC and Prevacid 24HR revealed that its labeling was virtually identical to that of Prilosec.8,9
Both products share label warnings and precautions that help ensure safe use.8,9 Both are contraindicated under the age of 18 and in patients who are pregnant or breastfeeding. They are only to be recommended when the patient has frequent heartburn, defined as heartburn occurring on 2 or more days per week. Neither will provide immediate relief of heartburn, as the patient may need to administer the product for 1 to 4 days before full relief is felt. However, their duration is longer than that of other product categories. It is worth noting that many patients do experience relief on the first day of administration.
Patients are directed to take 1 capsule/tablet with a glass of water before eating in the morning, for 14 consecutive days.8,9 This 14-day course of therapy must not be repeated more often than every 4 months. Patients should not use either product if they have trouble or pain swallowing food, bloody vomitus, or bloody or black stools. In these cases, a physician should be consulted. Patients should speak to a physician before using either product if they have had heartburn for more than 3 months; heartburn with lightheadedness, sweating, or dizziness; chest or shoulder pain with shortness of breath, sweating, or lightheadedness; pain spreading to the arms, neck, or shoulders; frequent chest pain; frequent wheezing, especially along with heartburn; unexplained weight loss; nausea or vomiting; or stomach pain. Prevacid 24HR warns patients with liver disease not to use it, a warning not required on labels of Prilosec OTC.8,9
Patients contemplating purchase of either product are directed to speak to their physician or pharmacist if they are taking warfarin, prescription antifungal or antiyeast medications, digoxin, or tacrolimus. However, there are some differences in labeling. Only Prilosec OTC carries drug interaction warnings with clopidogrel and diazepam, and only Prevacid 24HR carries a warning against use with theophylline. A minor difference is that Prilosec generally warns against use with prescription antivirals, while the label for Prevacid 24HR specifically mentions atazanavir.8,9
Patients should stop using these products if the heartburn continues or worsens, if they feel that they need to take them for more than the 14-day period recommended on the label, or if they need to take more than one course of treatment every 4 months.8,9
Zegerid OTC: Zegerid was switched to OTC status in 2009.2 It contains omeprazole, with added sodium bicarbonate to protect omeprazole from degradation by acidic gastric contents and to enhance absorption. Its labeling is almost identical to that of Prilosec OTC, with a few exceptions.10 It warns against use in patients who are on salt-restricted diets. Further, while it does not carry a warning against use in patients taking clopidogrel, it does carry a warning against use with any other prescription drug, adding the statement, “Sodium bicarbonate may interact with certain prescription drugs.”10 The label also cautions patients to take the product at least 1 hour before eating in the morning.
Zyrtec: Zyrtec (cetirizine) was the second nonsedating antihistamine, following Claritin (loratadine) by about 5 years.2 A close examination of both labels reveals some important therapeutic differences between the two.11,12 While both warn against unsupervised use if the patient has liver or kidney disease or is allergic to the product’s ingredients, Zyrtec also warns against use if the patient has an allergy to hydroxyzine. Further, while the label of Claritin warns against exceeding the recommended dose to prevent possible drowsiness, Zyrtec goes much further. Its label warns patients to speak to a physician or pharmacist before use if they are taking tranquilizers or sedatives. It also warns the patient that drowsiness may occur (apparently even with normal doses), that they must be careful when driving or operating machinery, that they should avoid alcohol, and that use of alcohol, sedatives, or tranquilizers may increase drowsiness.12 From these additional warnings, the pharmacist should infer that Zyrtec is far more prone to cause sedation than Claritin.
alli: Introduced in 2007, alli (orlistat) is the only FDA-approved OTC weight-loss product.2 Despite the overwhelming number of tablets, capsules, and liquids promising to promote weight loss, these products are of unknown safety and efficacy, including many dietary supplements, herbals, and homeopathics. Thus, alli is significant, being the sole product in its category. However, since patients may be concerned about possible gastrointestinal side effects, it is important for them to follow the product’s instructions carefully.13
Plan B One-Step: In 2006, Plan B was introduced as the first nonprescription morning-after (emergency) contraceptive.2 In 2009, Plan B One-Step debuted, and the original Plan B was discontinued. Plan B One-Step’s labeling is virtually the same as that of the original Plan B, with the exception that the patient takes only 1 tablet, which simplifies its use greatly.14
Future Switches
It is usually difficult to discover which products are being considered for future switches, as manufacturers’ marketing plans are highly confidential. However, there has been a sustained push to switch statins to OTC status.15 So far, the FDA has not allowed this, citing the potential for improper patient use and possible side effects

Common Questions About New Products
Rx-to-OTC product switches have occurred with Prilosec, Prevacid, Zyrtec, Claritin, Rogaine, and dozens of other popular nonprescription products. When these medications were Rx only, you had to visit a physician for a prescription and then travel to a pharmacy to have it filled. The pharmacist was required to provide you with the information needed for safe use. After the drug becomes available as an OTC product, however, it can be sold in retail stores by someone without any medical training, or even obtained from a vending machine. It is always better to purchase such products from a pharmacist who has the knowledge to advise you on how to use the product safely. In fact, although switched to nonprescription status, certain products, such as emergency contraceptives (e.g., Plan B One-Step), must be bought from behind the pharmacy counter.
Is This Product Safer Now That I Can Buy It Anywhere?
In order for the product to be switched to OTC status, the manufacturer was required to answer numerous questions about safety, perhaps even carrying out new studies. Nevertheless, the ingredients inside the product are the same as they were when it was Rx only, with similar dangers and potential for harm, although the dosage may be different. These switched products are considered safe for nonprescription use only because the OTC label helps ensure that you will use them under the exact conditions that help guarantee safety. Thus, the label is your guide to safe use, backed up by a pharmacist who can answer your questions.
Can I Use the OTC Product Instead of My Prescription?
Sometimes the same ingredient is available as a prescription drug and also in an OTC version. For instance, patients taking prescription Prilosec may notice that Prilosec OTC contains the same active ingredient (omeprazole). It is tempting to stop seeing your physician for the Rx drug and just buy the OTC product. However, there are numerous reasons not to do so without physician advice. As an example, you may have been prescribed Prilosec for an ulcer, a condition that requires careful monitoring. If you take the OTC version without returning to your physician, you may get worse. Your dose or dosage directions may be different from those on the OTC label. In these cases, you cannot use the OTC version as a substitute. For example, you should not use two of the 20 mg OTC tablets to duplicate the 40-mg dose of an Rx tablet, as that dose is not approved for self-care.
Do I Still Need to See a Doctor for This Problem?
The answer to this question depends on the nature of your problem. For example, as previously mentioned, you may use a product such as Prilosec OTC for heartburn, which is indicated on the label. However, ulcer is not indicated, so you should not use it for this condition unless a physician directs you to do so. Once again, your questions can be answered by paying close attention to the label or asking a pharmacist for further assistance.
REFERENCES
1. Pray WS. Nonprescription Product Therapeutics. 2nd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2006.
2. Now available without a prescription. FDA. www.fda.gov/Drugs/ResourcesForYou/Consumers/ucm143547.htm. Accessed August 26, 2010.
3. Lipsky MS, Waters T. The “prescription-to-OTC switch” movement. Its effects on antifungal vaginitis preparations. Arch Fam Med. 1999;8:297-300.
4. FAQs about Rx-to-OTC switch. Consumer Healthcare Products Association. www.chpa-info.org/scienceregulatory/FAQs_Switch.aspx. Accessed August 26, 2010.
5. Pray WS. Updates in nonprescription therapy for heartburn and GERD. US Pharm. 2009;34(10):52-55.
6. Ingredients and dosages transferred from Rx-to-OTC status by the FDA since 1975. Consumer Healthcare Products Association. December 2, 2009. www.chpa-info.org/media/resources/r_4620.pdf. Accessed August 26, 2010.
7. Rx-to-OTC switch list. FDA. www.fda.gov/AboutFDA/CentersOffices/CDER/ucm106378.htm. Accessed August 26, 2010.
8. Prilosec OTC. Procter & Gamble. www.prilosecotc.com/en_US/hcp/Pages/ClearLabeling.aspx?parentNode=About+Prilosec+OTC. Accessed August 26, 2010.
9. Prevacid 24HR. Drug facts and patient package insert. Novartis Consumer Health, Inc. http://Prevacid24hr.com/hcp/patient-package-insert.jsp. Accessed August 26, 2010.
10. Zegerid OTC product labeling. Schering-Plough HealthCare Products, Inc. www.zegeridotc.com/download/zegeridotc_product_labeling.pdf. Accessed August 26, 2010.
11. Claritin tablets. Schering-Plough HealthCare Products. www.claritin.com/claritin/products/ClaritinTablets.jspa. Accessed August 26, 2010.
12. Zyrtec. McNeil Consumer Healthcare. www.zyrtec.com/econsumer/zyrtec/pdf/Allergy_10mg_Tablet.pdf. Accessed August 26, 2010.
13. alli faqs. GlaxoSmithKline. www.myalli.com/faq.aspx. Accessed September 9, 2010.
14. Plan B One-Step. Pharmacists. Counseling your patients. Teva Women’s Health. www.planbonestep.com/plan-b-pharmacists/plan-b-counselling.aspx. Accessed August 26, 2010.
15. Childs D. Public not ready for over-the-counter statins. ABC News. December 11, 2007. http://abcnews.go.com/Health/Cholesterol/story?id=3984364&page=1. Accessed August 26, 2010.
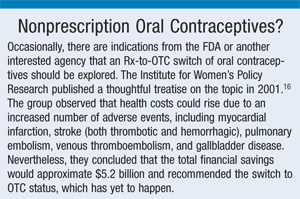
16. Mead H. Making birth control more accessible to women: a cost-benefit analysis of over-the-counter oral contraceptives. Institute for Women’s Policy Research. February 2001. www.iwpr.org/pdf/otc0201.pdf. Accessed August 26, 2010.
To comment on this article, contact rdavidson@uspharmacist.com.






