US Pharm. 2018:43(2)32-36.
ABSTRACT: Cardiovascular (CV) disease continues to be the number one cause of mortality in the United States. Diabetes mellitus, both type-1 and type-2 (T2DM), increases the risk of CV events, CV mortality, and cancer death. Many approaches improve CV disease outcomes in diabetes, including controlling hypertension, treating hyperlipidemia, using antiplatelet drugs in some patients, and achieving glycemic control. Defining the best evidence-based glycemic goals continues to be challenging and sometimes controversial. There is now encouraging evidence, as well as recent FDA approvals for specific diabetes drugs, including SGLT-2 inhibitors and GLP-1 agonists, to improve CV outcomes in patients with T2DM.
Cardiovascular (CV) disease remains the number one killer of both men and women in the United States.1 The most comprehensive study to date on cause of death in patients with diabetes analyzed 123,205 deaths among 823,900 individuals and found an increase in all-cause death of 1.8× (95% CI: 1.71-1.90); vascular death, 2.32× (95% CI: 2.11-2.56); cancer death, 1.25× (95% CI: 1.19-1.31); and other death, 1.73× (95% CI: 1.62-1.85).2 Complications of untreated or poorly treated type 1 and type 2 diabetes include, but are not limited to, CV problems.
The American Diabetes Association (ADA) published a joint scientific statement with the American Heart Association (AHA) in 2014 outlining the current understanding of CV outcomes in type 1 diabetes mellitus (T1DM). This article specifies hypertension as a strong risk factor for CV disease in T1DM and a moderate risk factor in type 2 diabetes mellitus (T2DM); in addition, hyperlipidemia and insulin resistance are very important contributors to CV disease in T2DM and moderate contributors in T1DM. Most strongly linked to CV disease in both T1DM and T2DM are microalbuminuria and poor glycemic control.3 Medications play an important part in modifying most of the contributors identified in the ADA/AHA statement. This article will focus on glycemic control and diabetes medications for reducing CV disease outcomes.
The United Kingdom Prospective Diabetes Study (UKPDS 33) showed that intensive glycemic control (usually with insulin or a sulfonylurea) resulted in a significant reduction (12%; P <.03) in all diabetes-related endpoints in patients with T2DM. Much of the total effect was attributable to a reduction in microvascular complications. An even more impressive diabetes-related endpoints reduction (24%; P <.005) was seen with intensive blood pressure control.4 Significant benefit persisted for 10 years after completion of the trial—with a 9% reduction in diabetes-related endpoints—in the intensive-treatment group, for those initially randomized to glucose-lowering therapy.5
Glycemic Targets and Glycemic Control
Determining an exact glycemic target to reduce CV events in patients with diabetes mellitus has been elusive, and suggested A1C percentage targets have been controversial. In the landmark diabetes trials (UKPDS, DCCT), more intensive interventions and lower A1C levels demonstrated improved outcomes. However, when much lower A1C targets have been attempted in clinical trials (e.g., <6%) the CV and mortality outcomes have been worse than with more relaxed targets (e.g., A1C 7.0%-7.9%).6 At least a portion of the increased mortality in the ACCORD trial is attributable to higher rates of severe hypoglycemia in the intensive-treatment group, and it is undetermined whether lower A1C targets may be beneficial if excess hypoglycemia is avoided.7 The most commonly accepted A1C goal for T1DM and T2DM in most patients, if hypoglycemia can be minimized, is <7.0%.
Insulin in T1DM
Untreated T1DM is ultimately fatal. Only exogenous insulin can sustain life in these patients, and other therapies can offer various additional, but adjunctive, benefits. Some major points from the ADA/AHA publication related to glycemic control and effects of insulin in patients with T1DM are paraphrased here:
• Early optimal glycemic control in T1DM produces long-term CV disease benefits.
• Obesity and associated insulin resistance in patients with T1DM is increasing.
• Outcomes data with modification of obesity or insulin resistance in patients with T1DM are limited.
• Some evidence suggests that weight gain in the presence of improved glycemic control is associated with a paradoxical reduction in the CV disease risk profile.3
It may be this simple: The best use of insulin in T1DM (and all medications when treating T1DM or T2DM) includes achieving appropriate glycemic control while minimizing hypoglycemia. Insulin-pump therapy was recently shown to be superior to sc insulin injections for this purpose, with lower rates of severe hypoglycemia and ketoacidosis, which was remarkable given the improved glycemic control with insulin pumps in children, adolescents, and young adults with T1DM.8 Meanwhile, for T2DM, the greatest evidence for specific treatment modifying CV outcomes derives from recent trials of relatively new drugs.
Specific Agents in T2DM
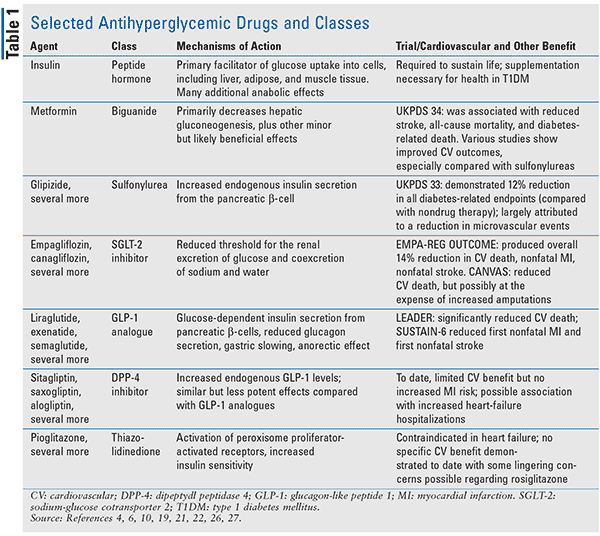
In contrast to T1DM, T2DM early in its natural history may not absolutely require insulin administration, and many treatment options exist. Several of the newest drugs for T2DM, including the sodium-glucose cotransporter 2 (SGLT-2) inhibitors and glucagon-like peptide 1 (GLP-1) analogues, have demonstrated an ability to decrease CV complications of T2DM; some have successfully acquired CV-protection FDA indications. An ongoing surge of clinical-trial CV data for medications used in T2DM indicates that more of these agents will likely be recognized for their ability to decrease the risk of CV complications. Reviewed below are metformin, SGLT-2 inhibitors, GLP-1 agonists, dipeptidyl peptidase 4 (DPP-4) inhibitors, and thiazolidinediones (TZDs) with respect to CV complications (Table 1).
Metformin
Metformin is ranked by guidelines as the first-line pharmacologic agent (if immediate insulin therapy is not required), in conjunction with lifestyle modifications, in most patients with T2DM without kidney dysfunction (preferably estimated glomerular filtration rate > 45mL/min) and no other contraindications. It is typically well tolerated, is relatively inexpensive, seldom causes hypoglycemia, and reduces A1C levels as well as any noninsulin drug. Notable concerns with metformin use include lactic acidosis and intolerable gastrointestinal upset.9
In UKPDS 34, the advantages and disadvantages of metformin for intensive glycemic control in overweight patients newly diagnosed with T2DM were specifically examined. Metformin therapy in these patients, compared with conventional therapy, produced impressive results, including a 32% reduction (P = .002) in any diabetes-related endpoint. Metformin was associated with reduced stroke (P = .032) and reduced all-cause mortality (P = .021); it also reduced diabetes-related death by 42% (P = .017). Metformin, compared with other therapies, including sulfonylureas and insulin, produced less weight gain and fewer hypoglycemic events. One concern arose in patients already taking maximum doses of sulfonylureas who were randomized to early (lacking symptoms of hyperglycemia) use; they experienced a 96% increase in diabetes-related death rates (P = .039). Further investigation did not find significant risk of diabetes-related death with the combination. In conclusion, metformin reduces stroke, all-cause mortality, and diabetes-related death compared with what was once called conventional therapy.10
An additional randomized, controlled trial of metformin and sulfonylureas is available. In this prospective, double-blind trial in 304 patients with T2DM and coronary artery disease, subjects randomized to metformin or glipizide for 3 years obtained similar glycemic control (average A1C of 7.0% and 7.1%, respectively). The patients given metformin sustained significantly fewer CV events (composite of CV death, nonfatal myocardial infarction (MI), nonfatal stroke, or arterial revascularization procedure). No significant difference in mortality was demonstrated between the groups. The reported hazard ratio for CV events for a median follow-up of 5 years was 0.54 (95% CI: 0.30-0.90, P <.03).11
SGLT-2 Inhibitors
SGLT-2 inhibitors are indicated for patients with T2DM who have not achieved goal A1C with lifestyle modification.12 Most guidelines recommend SGLT-2 inhibitors as add-on therapy to metformin although monotherapy is potentially appropriate.13-15 Use of this class of drugs has continued to increase yearly since market introduction because of their many positive effects, including low risk of hypoglycemia; compelling degrees of A1C reduction; osmotic renal excretion of glucose and the corresponding caloric loss; and modest blood pressure reduction.16,17 Use of these agents is limited by the risk of (normoglycemic) ketoacidosis, acute kidney injury, and genitourinary infections, as well as, for many, cost. Recent reports suggest an increased risk of lower-extremity amputations, in particular with canagliflozin, indicating that cautious use or avoidance in patients with peripheral vascular disease is wise.18
The EMPA-REG OUTCOME trial showed empagliflozin produced a relative risk reduction of CV mortality of 38% compared with placebo (P <.001) at median observation time of 3.1 years.19 This, in turn, is what drove an overall 14% reduction in the primary adverse CV endpoint of CV death, nonfatal MI, and nonfatal stroke. This impressive reduction in CV mortality should be interpreted with caution and may not apply to all T2DM patients because more than 99% of the patients enrolled in EMPA-REG OUTCOME had CV disease.19
The most recent published research on the CV benefits of an SGLT-2 inhibitor produced mixed results. The CANVAS trial showed that canagliflozin reduced CV events, but possibly at the expense of amputations. The initial primary-prevention arm of CANVAS showed a modest (and statistically nonsignificant) reduction in CV events; these patients were at risk for CV disease, but less than those in the aforementioned EMPA-REG OUTCOME trial. The more recently published secondary-prevention arm of CANVAS demonstrated a more robust relative risk reduction among patients who had experienced a prior CV event. Ultimately, CANVAS demonstrated that canagliflozin reduced CV death, nonfatal stroke, and nonfatal MI overall compared with placebo (26.9 vs. 31.5 per 1,000 patient-years; hazard ratio, 0.86; 95% CI, 0.75-0.97; P <.001 for noninferiority; P = .02 for superiority).20,21 EMPA-REG OUTCOME was not designed to capture amputation risk, so there is currently no head-to-head comparison of amputation risk with empagliflozin versus canagliflozin, and such interpretation is likely not possible owing to multiple factors confounding any comparison between trials.
GLP-1 Analogues
GLP-1 agents, which include liraglutide, exenatide, and semaglutide, among others, cause glucose-dependent insulin secretion from pancreatic beta cells. Their use has been consistently associated with weight loss to varying degrees, ranging from 1 kg to approximately 3.5 kg in different trials.22 Although considerable variation exists across trial results, GLP-1 agonists produce significant reductions in A1C. In particular, the DURATION-1 trial demonstrated a potent reduction in A1C of 1.8 points with weekly exenatide use and 1.4 points with twice daily exenatide dosing.23 Their use is limited by cost and high incidence of gastrointestinal side effects, including nausea and diarrhea. Additionally, acute pancreatitis, subclinical pancreatic inflammation, and pancreatic cancer have been reported with exenatide use.24
The LEADER trial showed that liraglutide significantly reduced risk of CV death, nonfatal MI, and nonfatal stroke at 3.8 years; the trial met all primary endpoints.25 The CV benefit is better established in patients with CV disease than in those without. Hypoglycemic and gastrointestinal side effects were significantly higher in the treatment group than in the placebo group. The LEADER trial included only high-risk CV patients, so the demonstrated CV benefit may be significantly diluted among the general T2DM population.
The SUSTAIN-6 trial showed that semaglutide significantly reduced the primary outcome of first nonfatal MI and first nonfatal stroke.26 However, rates of CV death were similar in treatment and placebo groups. SUSTAIN-6 is substantially limited in that it was not designed to detect superiority and was of relatively short duration (median 2.1 years).26
DPP-4 Inhibitors
DPP-4 inhibitors, including saxagliptin and alogliptin, are effective glucose-lowering agents that carry low risk of hypoglycemia and weight gain. Some CV benefit may be expected, given some similarity in action to the GLP-1 analogues; both classes of agents exert their effects through the incretin receptor system. The main purpose of recent FDA trials was to detect increased risk of CV events; the SAVOR-TIMI 53 and EXAMINE trials neither reduced nor increased CV death, nonfatal stroke, and nonfatal MI.27,28 However, saxagliptin—used in SAVOR-TIMI 53—showed an increased rate of heart-failure hospitalization compared with placebo, with the highest rates among those with preexisting chronic kidney failure and heart failure.29 There may be a not-yet-elucidated mechanism of atherosclerotic stabilization; this benefit was seen in early trials and does not appear to have been reproduced. This class of antihyperglycemics may not be ideal for use in high-risk CV populations; current FDA recommendations include discontinuing saxagliptin if heart failure develops.30
Thiazolidinediones
TZDs (pioglitazone, rosiglitazone) increase insulin sensitivity in peripheral tissues, in part through activating peroxisome proliferator-activated receptors. TZDs are contraindicated in patients with symptomatic heart failure and active liver dysfunction, and have alarming long-term side effects.31 The RECORD trial led to major FDA restrictions on rosiglitazone prescribing and distribution, owing to preliminary detection of CV risks. Several years later, a subsequent FDA adjudication removed the Risk Evaluation and Mitigation Strategy monitoring requirements. Despite improvement commonly seen in blood lipoproteins with TZD use, there is no evidence of specific CV benefits with TZDs, but they and other medications without particular FDA indications may benefit patients by helping them obtain glycemic control. Some researchers interpret an older single study to suggest that pioglitazone has significant CV benefits compared with rosiglitazone.32 However, the applicability, strength, and quality of this comparison are lacking compared with the higher-quality trial data now available for specific SGLT-2 inhibitors and GLP-1 agonists.
The Pharmacist’s Role
Pharmacists play a key role in educating patients and providers and making medication recommendations. Pharmacists can:
• Recognize hypoglycemia, help patients manage it, and encourage them to discuss measures with their prescriber to avoid it—including the use of medications with lower risk of hypoglycemia
• Help patients achieve glycemic control by assisting with ideal medication choice and selecting an individualized, appropriate A1C target to optimize CV outcomes in diabetes
• Identify and mitigate causes of nonadherence to diabetes medications
• Recommend diabetes medications with the best evidence and FDA indications to improve CV outcomes in patients with or at elevated risk for CV disease, or in patients particularly wanting to minimize CV disease
Conclusion
Evidence has been building for many years in support of the vascular benefits provided by medications in the treatment of diabetes. The new FDA regulatory requirement to ensure that new diabetes medications are not associated with increased CV risk has produced intriguing findings. Not only have some newer drugs demonstrated a degree of relative CV safety, certain drugs have demonstrated significant improvement in CV outcomes. It is important to appropriately generalize this information to groups of patients that fit the populations in these studies. These data provide a renewed hope for improved outcomes for patients with diabetes and a clear incentive for patients to take these medications as prescribed.
REFERENCES
1. CDC. Health, United States, 2016. Table 19. www.cdc.gov/nchs/data/hus/hus16.pdf#019. Accessed November 25, 2017.
2. Seshasai SR, Kaptoge S, Thimpason A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829-841.
3. Ferranti SD, de Boer IH, Fonseca V, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2014;37:2843-2863.
4. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853.
5. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
6. Gerstein HZ, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes: The Action to Control Cardiovascular Risk in Diabetes [ACCORD] Study Group. N Engl J Med. 2008;358:2545-2559.
7. Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909.
8. Karges B, Schwandt A, Heidtmann B, et al. Association of insulin pump therapy vs insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes. JAMA. 2017;318(14):1358-1366.
9. American Diabetes Association. Standards of medical care in diabetes – 2017. Diabetes Care. 2017;40:S1.
10. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854-865.
11. Hong J, Zhang Y, Lai S, et al. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care. 2013;36(5):1304-1311.
12. FDA. Center for Drug Evaluation and Research. Drug safety and availability—sodium-glucose cotransporter-2 (SGLT2) inhibitors. Published May 16, 2017. www.fda.gov/Drugs/DrugSafety/ucm446852.htm. Accessed November 23, 2017.
13. Mikhail N. Place of sodium-glucose co-transporter type 2 inhibitors for treatment of type 2 diabetes. World J Diabetes. 2014;5(6):854.
14. Type 2 diabetes in adults: management, NICE guideline NG28. Published December 2015. www.nice.org.uk/guidance/ng28/resources/algorithm-for-blood-glucose-lowering-therapy-in-adults-with-type-2-diabetes-2185604173. Accessed November 23, 2017.
15. Fung CSC, Wan EYF, Wong CKH, et al. Effect of metformin monotherapy on cardiovascular diseases and mortality: a retrospective cohort study on Chinese type 2 diabetes mellitus patients. Cardiovasc Diabetol. 2015;14:137.
16. Chaplin S. Primary care diabetes prescribing rates: latest analysis shows continued rise in volume and cost. Practical Diabetes. Published January 31, 2017. www.practicaldiabetes.com/article/primary-care-diabetes-prescribing-rates-latest-analysis-shows-continued-rise-volume-cost/. Accessed November 24, 2017.
17. Minze MG, Will K, Terrell BT. Benefits of SGLT2 Inhibitors beyond glycemic control – a focus on metabolic, cardiovascular, and renal outcomes. Curr Diabetes Rev. 2017;13; Epub ahead of print.
18. FDA. Center for Drug Evaluation and Research. Drug Safety and Availability—FDA Drug Safety Communication: FDA confirms increased risk of leg and foot amputations with the diabetes medicine canagliflozin (Invokana, Invokamet, Invokamet XR). Published May 16, 2017. www.fda.gov/Drugs/DrugSafety/ucm557507.htm. Accessed November 23, 2017.
19. Zinman B, Wanner C, Lachin JM. et al. Empagliflozin, cardiovascular outcomes and mortality in type 2 diabetes. N Engl J Med. 2015;373(11):2117-2128.
20. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in Type 2 diabetes. N Engl J Med. 2017;377(7):644-657.
21. Mahaffey KW, Neal B, Perkovic V, et al. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation. November 2017:Epub ahead of print.
22. Shah M, Vella A. Effects of GLP-1 on appetite and weight. Rev Endocr Metab Disord. 2014;15(3):181-187.
23. Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2015;6(1):19-28.
24. Singh S, Chang H-Y, Richards TM, et al. Glucagonlike peptide 1–based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus. JAMA Intern Med. 2013;173(7):534-539.
25. Marso SP, Daniels GH, Brann-Franzen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Eng J Med. 2016;375(18):311-322.
26. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
27. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327-1335.
28. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and CV outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
29. Scirica BM, Braunwald E, Raz I, et al. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130(18):1579-1588.
30. FDA. Center for Drug Evaluation and Research. Drug safety and availability – FDA drug safety communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. Published April 5, 2016. www.fda.gov/Drugs/DrugSafety/ucm486096.htm. Accessed November 25, 2017.
31. FDA. Center for Drug Evaluation and Research. Postmarket drug safety information for patients and providers - information for healthcare professionals: pioglitazone HCl (marketed as Actos, Actoplus Met, and Duetact). Published August 2013. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124178.htm. Accessed November 26, 2017.
32. Boyle PJ, King AB, Olansky L, et al. Effects of pioglitazone and rosiglitazone on blood lipid levels and glycemic control in patients with type 2 diabetes mellitus: a retrospective review of randomly selected medical records. Clin Ther. 2002;24(3):378-396.
To comment on this article, contact rdavidson@uspharmacist.com.