US Pharm. 2024;2024;49(7):39-43.
ABSTRACT: Ankylosing spondylitis (AS) is a chronic inflammatory disease that affects the spine and is characterized by stiffness and pain. AS impacts approximately 0.1% to 0.5% of the population and often presents with variable severity and extraarticular manifestations, such as inflammatory bowel disease and psoriasis. Diagnosis relies on medical history, physical examination, laboratories, and imaging, with treatment options including nonsteroidal anti-inflammatory drugs and tumor necrosis factor inhibitors. Pharmacists are integral in choosing, dosing, and managing AS medications, as well as addressing medication-accessibility issues and accommodating patient preferences. Interprofessional collaboration is crucial for optimizing care, emphasizing the need for personalized approaches to improve patient outcomes and quality of life.
Ankylosing spondylitis (AS) is a subset of arthritis that is characterized by chronic inflammation of the axial spine, resulting in arthralgia, stiffness, and limited flexibility.1-3 This condition impacts roughly 0.1% to 0.5% of the population, with variable presentation in terms of symptom severity and frequency.3 Hallmark features of AS include chronic back pain and progressive spinal stiffness with involvement of the sacroiliac (SI) joints and peripheral joints, digits, and entheses.2,4,5 AS often leads to impaired spinal mobility and can result in postural abnormalities. In addition to skeletal involvement, AS can have extraarticular manifestations, including inflammatory bowel disease (IBD), psoriasis, and uveitis, with the most common of these sequelae being IBD, impacting nearly 50% of AS patients.2,3,6 Other complications include an increased risk for cardiovascular (CV) disease associated with systemic inflammation, restrictive pulmonary complications related to reduced chest wall rise and limited spinal mobility, and an increased risk for vertebral fragility fractures.2,3
Causes and Diagnosis
AS is a chronic inflammatory disease that typically presents gradually and without obvious early symptoms. While the cause of AS is largely unknown, research suggests that genetic and environmental factors contribute to disease development.2 Enthesitis, the primary pathology, is a chronic inflammation involving infiltration of immune cells, including helper and cytotoxic T cells, macrophages, cytokines, tumor necrosis factor-α (TNF-α), and transforming growth factor-β (TGF-β), which contribute to the inflammation, fibrosis, and ossification at sites affected by enthesitis.2,5 Risk factors for AS include family history, age younger than 45 years, and the presence of other inflammatory conditions, such as Crohn’s disease, ulcerative colitis (UC), or psoriasis.6 Expression of human leukocyte antigen (HLA)-B27 appears to be the most prominent genetic factor linked to AS prevalence, with approximately 5% to 6% of AS patients testing positive for this genetic marker.2 However, the presence of this gene does not guarantee AS, demonstrating the role of environmental factors.
The diagnosis of AS is determined by medical history, physical examination, laboratories, and imaging. If AS is suspected, a comprehensive history and physical examination should be completed. Gradual inflammatory back pain that improves with exercise, as well as spinal stiffness, limited mobility, and postural changes such as pronounced forward curvature of the spine, are common among patients with AS.2 Laboratory values, including elevated acute-phase reactants such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), are typically nonspecific for the diagnosis of AS but may provide supportive evidence. Imaging remains the primary diagnostic tool for AS. Additional imaging with MRI may be useful to inform the shared decision-making process or to evaluate disease progression but should be avoided at scheduled intervals to reduce radiation exposure.3
Treatment Options
In AS treatment, the first course of action is to determine disease activity level. Active disease refers to disease causing unacceptable symptoms, while stable disease refers to asymptomatic disease or an acceptable level of symptoms.3 Once the disease activity level has been determined, pharmacists can play an important role in pharmacologic treatment selection, dosing, and monitoring. Treatment for AS includes a combination of nonpharmacologic and pharmacologic modalities per the current 2019 American College of Rheumatology Treatment Guidelines for AS (see TABLE 1 and FIGURE 1). The goal of treatment is to alleviate symptoms, improve the ability to complete activities of daily living, decrease disease complications, and prevent further skeletal damage.3
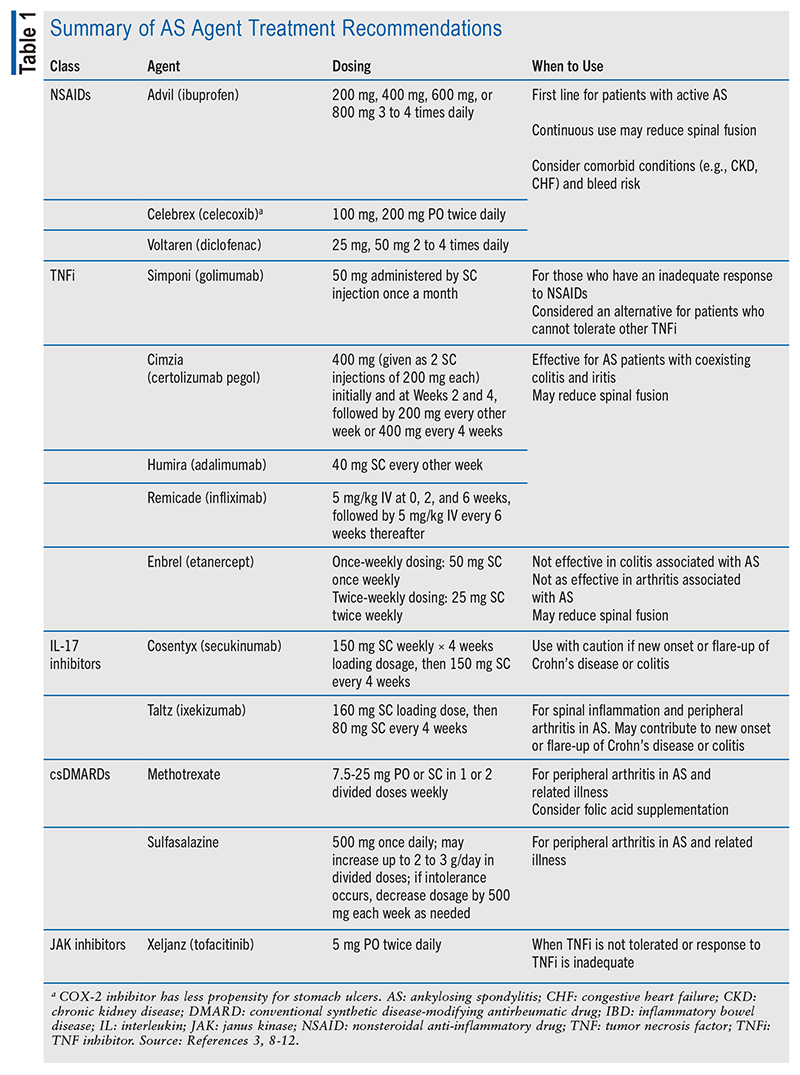
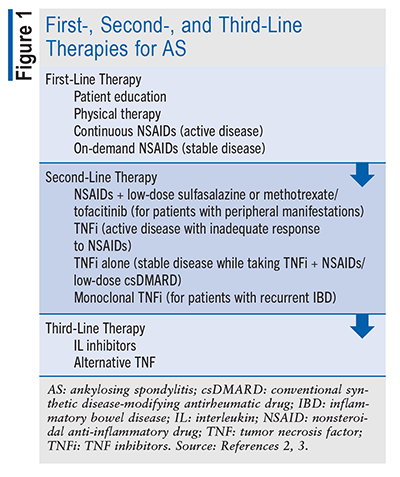
Active Disease: Nonsteroidal anti-inflammatory drugs (NSAIDs) remain the first-line therapeutic option for patients with active AS.3 Current guidelines recommend continuous use of NSAIDs over on-demand use or no treatment with NSAIDs for patients with active AS; however, no agent has been cited as more efficacious than another.3 A patient’s response to NSAIDs should be assessed 4 to 6 weeks after initiation. In patients with active AS despite treatment with NSAIDs, tumor necrosis factor inhibitors (TNFi) should be considered as the next therapeutic addition before interleukin (IL)-17 inhibitors, janus kinase inhibitors, or conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), such as methotrexate or sulfasalazine.3 There is no preferred TNFi except for patients with active AS and IBD or uveitis, in which case, adalimumab and infliximab are preferred over entanercept.3 Sulfasalazine and methotrexate should only be considered as a second-line option in patients with active AS with prominent peripheral arthritis or if a TNFi is not available.3
In patients with active AS who are on a TNFi, cotreatment with methotrexate or sulfasalazine is not recommended. Patients’ response to TNFi should be assessed 12 weeks after initiation. Instead, primary nonresponders (those who have not had a meaningful response within 3 to 6 months that is not associated with toxicity or lack of adherence) should be initiated on an IL-17 inhibitor preferentially over tofacitinib. For secondary nonresponders who have had a recurrence of AS activity despite appropriate treatment (generally 6 months), the guidelines conditionally recommend the use of another TNFi over the use of a non-TNFi biologic.3 A biosimilar of the first TNFi should not be used in primary or secondary nonresponders. Additionally, systemic glucocorticoids should be avoided for treatment of active AS.3
For patients with additional disease manifestations, other treatment modalities may be needed. Patients experiencing additional disease manifestations, including peripheral-predominant arthritis, stable axial disease and active peripheral arthritis, or stable axial disease and active enthesitis despite appropriate NSAID use, may benefit from locally administered parenteral glucocorticoids over no treatment.3
Due to a lack of evidence supporting the benefit of a treat-to-target strategy, as seen in other inflammatory conditions, the guidelines conditionally recommend against this use to decrease burden on patients and providers.3 In addition to appropriate pharmacotherapy, patients with active AS should receive land-based (as opposed to aquatic) physical therapy, with a preference for active modalities (supervised exercise) over passive interventions (ultrasound, heat, massage).3 The goal of pharmacologic and nonpharmacologic strategies is to improve quality of life and prevent further skeletal damage.3
Stable Disease: In patients with stable AS who are not currently on any pharmacologic treatment, rather than treating with continuous use, it is recommended that these individuals use NSAIDs on demand for short-term flares and recurrent symptoms. Again, no NSAID has been proven to exhibit greater efficacy over another.3 For patients with stable AS who are being treated with TNFi and NSAIDs or csDMARDs, it is recommended to continue only the TNFi; however, these patients can still use NSAIDs as needed for occasional symptoms.3
A patient with stable AS who is being treated with TNFi or IL-17 inhibitors should generally continue his or her current treatment regimen to avoid relapse. However, if the patient has maintained prolonged stability, the dosage of the biological agent may be tapered provided that the patient and his or her healthcare team understand the risks and benefits of this adjustment. This does not mean tapering completely off the drug but rather reducing the dosage while still continuing treatment.3
Similarly, patients with stable AS who are being treated with an originator TNFi should continue treatment with their current regimen rather than switching to a biosimilar or interchangeable biologic. Currently, there are no established benefits for changing a patient’s biologic regimen during management of stable AS. Further studies are necessary to understand the impact and potential complications of switching from an originator TNFi to a biosimilar or interchangeable agent.3
The recommended agents for active or stable AS management are impacted by a patient’s comorbidities. For patients with recurrent uveitis or IBD, the use of TNFi monoclonal antibodies is recommended over other agents. Adalimumab and infliximab are preferred TNFi monoclonal antibodies for patients with recurrent uveitis, although certolizumab or golimumab may also be considered despite less available data. Likewise, adalimumab and infliximab may also lower the risk of IBD exacerbation in patients with AS. Specifically, adalimumab, infliximab, and certolizumab are recommended for treatment of Crohn’s disease, while adalimumab, infliximab, and golimumab are recommended for treatment of UC. Secukinumab, ixekizumab, and etanercept are not recommended over TNFi monoclonal antibodies in patients with AS and IBD due to studies reporting their association with increased risks of IBD exacerbations.3 Ultimately, the choice of biological agent for patients with AS and IBD should be determined with the patient’s gastroenterologist.
The Pharmacist’s Role
AS is a systemic disorder; therefore, an interprofessional team approach is recommended to provide comprehensive care. A traditional interprofessional team may consist of rheumatologists, who typically play the primary role in diagnosis and disease management, as well as primary care providers, gastroenterologists, ophthalmologists, cardiologists, physical and occupational therapists, pharmacists, and nurses.2 Pharmacists, particularly in the ambulatory care, community, and managed care settings, are strategically placed to play active roles in drug procurement and therapeutic management of patients with AS. Given that many patients with AS will utilize specialty medications, including TNFi and IL-17 inhibitors, managed care pharmacists’ involvement in drug procurement may help navigate insurance barriers and assist patients with high out-of-pocket costs.7 Furthermore, clinical management activities include ensuring appropriate baseline screening and vaccine administration, providing drug-specific patient education, and performing routine follow-up and assessment.
Patient Education and Counseling
Patient education is one of the most crucial areas where pharmacists can have a direct impact on the overall clinical management of patients’ treatment of AS. Patients should be counseled about the dosing, administration, most common and severe adverse effects, storage and disposal, and expectation of the drug’s effect each time a medication is initiated.7
NSAIDs have been associated with various adverse effects involving the gastrointestinal (GI), renal, and CV systems. Common adverse effects of NSAIDs include symptoms related to the upper GI tract, such as nausea, pyrosis, and dyspepsia. More severe symptoms are related to the lower GI tract, such as a lesion, and may go undetected. Pharmacists should counsel patients who are prescribed NSAIDs for continuous use to recognize the signs and symptoms of lower GI tract complications, such as nausea, vomiting, dizziness, fatigue, abdominal cramping, or stools that may be bright red or black and tarry. Patients should be advised to seek immediate medical attention if a GI bleed is suspected, as this can be a life-threatening complication.
The dose-dependent relationship between NSAIDs and renal adverse effects has been well documented. In patients without comorbidities, adverse effects such as acute kidney injury (AKI) or fluid and electrolyte retention are rare; however, patients with a history of hypertension, diabetes, or heart failure are at a higher risk of developing renal complications with NSAID use. In particular, chronic NSAID use may not only lead to an AKI but also increase patients’ risks for chronic kidney disease (CKD) and acute interstitial nephritis. Although uncommon, patients should still be counseled to recognize symptoms of renal side effects, including reduced urine output, edema due to fluid retention, excess thirst, and altered mental status. These NSAID-induced renal complications may also lead to CV effects, such as hypertension, myocardial infarction, and cerebrovascular accidents. Patients should be made aware of these risks, especially if they are prescribed continuous NSAID use for active AS or have comorbidities that increase their likelihood for developing these adverse effects.8
Methotrexate, sulfasalazine, TNFi, and IL-17 inhibitors are all associated with a high risk of serious infections due to their immunosuppressant properties. Infections may be bacterial, viral, or fungal in nature and may cause opportunistic infections such as herpes zoster, candidiasis, and pneumocystis pneumonia. Reactivation of latent tuberculosis and hepatitis B or C may occur a few months after treatment initiation. Patients should be screened for these latent infections prior to starting an immunosuppressant treatment for AS and stay up to date on recommended vaccinations. They should be encouraged to seek medical care should systemic symptoms of an infection develop, such as fever, fatigue, chills, malaise, and myalgia.9-11
Patients must be counseled on the association between TNFi and malignancy secondary to its myelosuppressive effects, particularly lymphoma or nonmelanoma skin cancer. Caution should be used in those with an elevated risk or history of malignancy, and all patients should be encouraged to adhere to recommendations regarding cancer screenings to manage these complications early should they develop. While there is a lack of data surrounding IL-17 inhibitors and malignancy, patients should be screened regularly for cancer since the risks are not yet fully understood.10,11
Agents that are used to treat AS have other unique side effects in addition to infection and malignancy. Methotrexate may cause alopecia, interstitial lung disease, and folic acid deficiency, while sulfasalazine is associated with GI distress and rare incidences of drug reactions with eosinophilia and systemic symptoms or drug rash with eosinophilia and systemic symptoms syndrome. TNFi commonly result in minor adverse effects such as headache, cough, diarrhea, nausea, abdominal pain, and anemia, but these effects usually do not warrant discontinuation of the drug. They are also associated with exacerbations and increased hospitalizations in patients with heart failure, so caution must be exercised when considering TNFi in these patients. Studies have reported that IL-17 inhibitors not only exacerbate IBD but also contribute to its development. Patients should be made aware of the signs and symptoms indicative of IBD and inform their doctor if they have a personal or family history of Crohn’s disease or UC.3,9-11
General counseling points include proper injection techniques for those agents that are administered subcutaneously, such as IL-17 inhibitors and most TNFi. Patients should disinfect the skin with an alcohol swab, choose an appropriate site for administration, and rotate injection sites after each use, among other important counseling points that are outlined in the package insert of each agent. Proper injection techniques will help alleviate the risk for injection-site reactions that may cause symptoms such as pain, swelling, pruritus, and erythema. With any new medication initiated, regardless of the drug class or route of administration, patients should be counseled on the signs of allergic reactions, including rash, hives, pruritus, swelling, and anaphylaxis, and seek medical care if these symptoms arise.10,11
Treatment Challenges
Treatment of AS involves several complexities. One challenge that may be encountered early in a patient’s treatment is weighing the risks and benefits of continuous NSAID use. Many patients in the United States will have a comorbidity or medication regimen that will exacerbate the GI, renal, and CV effects associated with NSAIDs, such as CKD, heart failure, or the use of antithrombotics and antihypertensives. These patients may need to utilize csDMARDs or TNFi as first-line therapy for AS. Even if a patient is otherwise healthy and does not take medications for chronic conditions, he or she may experience minor bothersome GI effects; however, these may be effectively managed with OTC treatments such as histamine-2 receptor blockers or proton pump inhibitors. For patients with susceptibilities for lower GI complications, misoprostol may also be utilized to lower this risk.8
Accessibility to TNFi and IL-17 inhibitors may also be a barrier to receiving optimized care for AS. While these treatments are effective, their substantial cost might pose a challenge for some patients. Additionally, insurance coverage for these agents may be limited or require extensive paperwork or prior authorizations that further delay treatment initiation. Being transparent with patients about the price of biologics is vital to promote shared decision-making processes regarding their treatment. Both providers and patients should be encouraged to investigate cost-saving strategies, such as patient-assistance programs, manufacturer coupons, or the utilization of biosimilars, if available.
Patient preferences should be acknowledged when initiating treatment for AS. Many patients are hesitant to use injectable medications or adhere to a strict physical therapy or exercise regimen. This is an opportunity for pharmacists to encourage individuals who show reluctance by informing them of the benefits that these treatments may have on their chronic pain and disease progression. Proper counseling and demonstration of injectable agents may ease patients’ apprehensiveness regarding their treatment.
Conclusion
AS is a rare, complex condition requiring prompt diagnosis and treatment to optimize patient outcomes. Pharmacists play an important role in helping maximize treatment outcomes through assistance with patient diagnosis, pharmacotherapy recommendations, and appropriate treatment adjustments. Examples of important interventions that pharmacists can make include identifying patients who may need modifications in pharmacotherapy based on their disease activity, recommending alternative agents for patients with contraindications, and managing drug interactions. Beyond these interventions, pharmacists also serve as valuable educators and advocates for patients with AS. They provide crucial information regarding treatment options, address patient concerns, and ensure appropriate administration techniques for injectable agents. Additionally, pharmacists can collaborate with the healthcare team to optimize treatment, monitor safety and efficacy, and promote adherence. By offering individualized support, empowering patients with knowledge, and fostering a patient-centered approach to care, pharmacists contribute significantly to improving outcomes and enhancing the quality of life for individuals with AS.
REFERENCES
1. Proft F, Poddubnyy D. Ankylosing spondylitis and axial spondyloarthritis: recent insights and impact of new classification criteria. Ther Adv Musculoskelet Dis. 2018;10:129-139.
2. National Institute of Arthritis and Musculoskeletal and Skin Diseases. Ankylosing spondylitis. www.niams.nih.gov/health-topics/ankylosing-spondylitis. Accessed April 10, 2024.
3. Ward MM, Deodhar A, Gensler LS, et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019;71(10):1599-1613.
4. Watad A, Cuthbert RJ, Amital H, McGonagle D. Enthesitis: much more than focal insertion point inflammation. Curr Rheumatol Rep. 2018;0;20(7):41.
5. Bridgewood C, Watad A, Cuthbert RJ, McGonagle D. Spondyloarthritis: new insights into clinical aspects, translational immunology and therapeutics. Curr Opin Rheumatol. 2018;30:526-532.
6. Wang R, Ward MM. Epidemiology of axial spondyloarthritis: an update. Curr Opin Rheumatol. 2018;30(2):137-143.
7. Mullican KA, Francart SJ. The role of specialty pharmacy drugs in the management of inflammatory diseases. Am J Health Syst Pharm. 2016;73(11):821-830.
8. Harirforoosh S, Asghar W, Jamali F. Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci. 2013;16(5):821-847.
9. Benjamin O, Goyal A, Lappin SL. Disease-modifying antirheumatic drugs (DMARD). In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024 Jan-. www.ncbi.nlm.nih.gov/books/NBK507863/.
10. Gerriets V, Goyal A, Khaddour K. Tumor necrosis factor inhibitors. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024 Jan-. www.ncbi.nlm.nih.gov/books/NBK482425/.
11. Eshwar V, Kamath A, Shastry R, et al. A review of the safety of interleukin-17A inhibitor secukinumab. Pharmaceuticals (Basel). 2022;15(11):1365.
12. Spondylitis Association of America. Spondyloarthritis medications overview. https://spondylitis.org/about-spondylitis/treatment-information/medications/guide-to-medications/. Accessed April 10, 2024.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





