US Pharm. 2020;45(4):32-36.
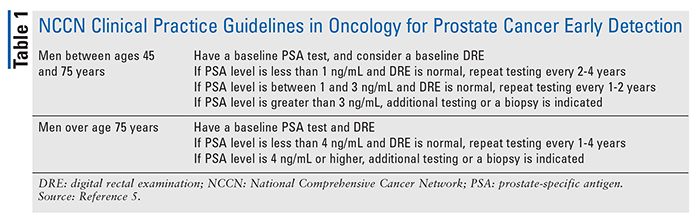
Prostate cancer develops when cells in the prostate gland become malignant.1 There are no hallmark signs and symptoms. Therefore, routine screening is imperative; practice guidelines for early detection can be found in TABLE 1. If left untreated, cancer cells may metastasize from the prostate to other parts of the body, specifically the bones and lymph nodes. Prostate cancer is confirmed by a biopsy.
Epidemiology
According to the National Cancer Institute, an estimated 174,650 new cases of prostate cancer would be diagnosed in 2019, accounting for 9% of new cancer cases in men.2 Researchers estimate prostate cancer to account for 31,620 deaths in 2019, which represent 5.2% of male cancer deaths.2 The age-adjusted death rates from prostate cancer declined by 51% from 39.08 deaths per 100,000 people in 1993 to 19.2 deaths per 100,000 people in 2014.2,3 The decreasing and comparatively low death rate, 3.8% of all deaths caused by cancer in men, suggest that increased public awareness with earlier detection and treatment has achieved a relative risk reduction (RR 0.84; 95% CI 0.73 to 0.95) in total mortality.3,4
Approximately one in nine men will be diagnosed with prostate cancer during his lifetime.3 The 5-year survival rate in the United States for men diagnosed with prostate cancer is 98%.2 Risk factors for prostate cancer include age, family history, and ethnicity.5 Men over the age of 50 years are at a higher risk.1 Men whose fathers or brothers have prostate cancer also have a two to three times higher risk of prostate cancer than men without a family history of the disease.3 Prostate cancer occurs more often in African American men than those of other races.5
Screening Methods
A prostate-specific antigen (PSA) test or digital rectal examination (DRE) is used to signal whether further testing should take place. A DRE is conducted by a physician by inserting a gloved and lubricated finger into the rectum to examine the prostate for any irregularities in size, shape, and texture.6 The PSA is a protein produced by the prostate, which is normally secreted into the ducts of the prostate.1 Conditions such as prostatitis, enlarged prostate, or prostate cancer elevate PSA to a level where it can be easily detected in the blood.6
A PSA level of 4 nanograms or higher per milliliter of blood is indicative that further testing is needed.7 If PSA is high, a biopsy will determine whether prostate cancer is present. During the biopsy, a physician obtains tissue sample from the prostate via the rectum and examines the specimen for cancerous tissues.8
Sangia Total PSA Test
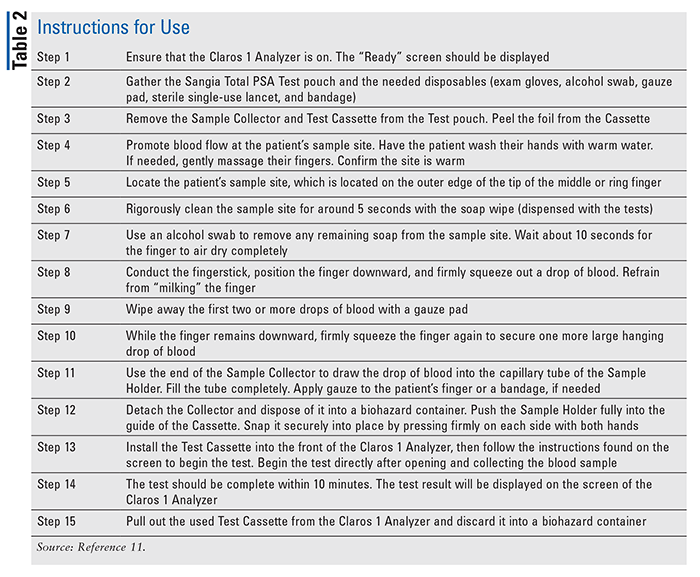
The Sangia Total PSA Test, developed by OPKO Health, Inc., was approved by the FDA on January 30, 2019. The test should be used in conjunction with a digital rectal exam to help detect if a patient has prostate cancer.9,10 The Sangia Total PSA Test is a “microfluidic-based point-of-care (POC) immunoassay for quantitative measurement of total PSA from whole blood utilizing Silver Amplified NeoGold ImmunoAssay (Sangia) technology.”9 With the prick of a finger, this device can determine a patient’s total PSA level within 10 minutes.11
The Sangia Total PSA Test box includes 20 individual tests stored in sealed pouches, a Lot Data Card with specific calibration data, 23 soap wipes (water and sodium lauryl sulfate), and the package insert.9 The Sangia Total PSA Test includes a Sample Collector and Cassette Assembly (Figure 1). The Sample Collector includes NeoGold-labeled anti–PSA antibodies, anticoagulant, buffers, protein stabilizers, and surfactants in a lyophilized formulation.9 The Cassette Assembly includes monoclonal anti–PSA antibodies, 45 microliters (μL) of silver salt, 45 μL of a reducing agent in acidic buffer, less than 5 μL of buffers with surfactants, protein stabilizers/blockers, and preservatives.9

This test is used in combination with the Claros 1 Analyzer (Figure 1).11 The blood sample is collected from a finger stick with the Sample Collector. The sample is collected by a medical professional certified in Clinical Laboratory Improvement Amendments (CLIA) for moderate-complexity testing, procedures that require basic laboratory knowledge and training, at the point-of-care testing site.11,12 The Sample Collector contains anti–PSA antibodies.11 The total PSA binds to these antibodies, creating an antigen-antibody complex.11 The Sample Collector is subsequently connected to the Cassette Assembly, and both are inserted into the Claros 1 Analyzer.11 Each sample mixture transferred from the Sample Collector to the cassette must conclude a separate positive and negative control check before the Claros 1 Analyzer can report a result.9 The user may input the patient’s information into the analyzer and, within 10 minutes, receive the results. The instructions for use can be found in TABLE 2.
Clinical Research and Effectiveness Results
OPKO Health, Inc., conducted a multicenter clinical study to evaluate the clinical validity and reliability of the Sangia Total PSA Test in the detection of prostate cancer in men aged 50 years and older. The primary outcome was based on sensitivity and specificity analysis. Sensitivity and specificity data can be found in TABLE 3. Eligible participants were men with no previous biopsy and no past medical treatment for benign prostatic hypertrophy 3 months prior to the study, except for tamsulosin and silodosin. Of 434 patients, the median age of all patients was 65.1 years, and the majority (85%) were Caucasian.11 A sample of blood collected from a fingertip was assayed using the Sangia Total PSA Test. No follow-up was necessary or required. The study indicated that a cutoff of 4.0 ng/mL was established to determine the performance of the test. The sensitivity of the Sangia Total PSA Test was 85.4% (95% CI, 0.80-0.89), and the clinical specificity was 30.3% (95% CI, 0.24-0.37).11 When combined with DRE, the clinical sensitivity increased by 59.2%, while the sensitivity of DRE alone is 31.8%. The clinical specificity of the Sangia Total PSA Test in conjunction with DRE decreased 61.7% compared with DRE alone.11 Therefore, the data support the use of the device in conjunction with DRE as an aid in the diagnosis of prostate cancer.
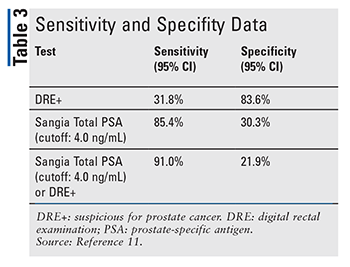
The Sangia Total PSA Test eliminates the long waiting periods by reducing waiting time to 10 minutes.12 The blood is collected using a capillary blood sample from a fingerstick, whereas traditional total PSA tests require testing in a central laboratory equipped to exclusively perform laboratory assessments, with results available in days or weeks.12 This point-of-care testing allows physicians and patients to have a discussion, during their current scheduled appointment, concerning further testing that includes a prostate biopsy and/or active surveillance of suspected disease. Furthermore, the test can be repeated to ensure accuracy and provide reproducible results within the same hour. OPKO calculated that using the test with DRE resulted in the detection of three times as many (91%) cancers as the rectal examination alone (31.8%).11
The test does not account for identifying asymptomatic tumors of the prostate or false-positive results, which can lead to overdiagnosis, anxiety, and stress.13 Despite the 85.4% sensitivity of the test, the clinical trial contained a high false-positive rate, 30.3% with 95% CI, reflecting the fact that the Sangia Total PSA does not account for factors that may increase or decrease the PSA level.11 This test does not negate the need for an uncomfortable DRE or an invasive biopsy for prostate cancer diagnosis. Also, the easy accessibility might promote the screening of men who do not require the test.11,12
CLIA requirements for testing with the Sangia Total PSA increases federal regulation of the testing site.13 OPKO is in the process of obtaining a CLIA waiver for the test and analyzer, which would permit the test to be performed by most medical office personnel with minimal training.13 If repeat testing is required, more samples must be retrieved from the patient, possibly causing discomfort. Also, its restricted use to aid in prostate cancer limits its use in the urology setting.12 The test will mainly be used in primary care settings where many patients complete their regular labs.12 U.S. federal law restricts the sale and point-of-care testing to physicians and clinical laboratories, and use is restricted to, by, or on the order of a physician.9 Patients will not be allowed to conduct this test at home.9
According to the Code of Federal Regulations Title 42 Chapter IV Section 493.1423 Standard, personnel performing moderate complexity testing must possess a license issued by the same state where the laboratory is located and, at a minimum, have a high school diploma (or equivalent) and documented training appropriate for the testing.14
Cost of the Sangia Total PSA Test
The cost of the Sangia Total PSA point-of-care testing has not been disclosed. Necessary parts such as the Claros 1 instrument and a single cassette are $2,500 and $15, respectively.15 The test is covered using the Physician Payments Sunshine Act under the Affordable Care Act and its implementing regulations, which is enforced through the Physicians Open-Payments Program.16
Conclusion
The Sangia Total PSA Test has demonstrated evidence of effectiveness and safety as an aid in the detection of prostate cancer in men aged 50 years and older in point-of-care settings. However, it should not be used alone to diagnose prostate cancer and should accompany an assessment of symptoms, clinical evaluation, DRE, and other laboratory tests or imaging techniques. An ultrasound-guided biopsy of the prostate must be performed to diagnose prostate cancer.
Additional information regarding the Sangia Total PSA Test can be obtained from the OPKO website at www.opko.com.17
REFERENCES
1. Norris LB, Kolesar JM. Prostate cancer. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 10th ed. New York, NY: McGraw-Hill. http://accesspharmacy.mhmedical.com/content.aspx?bookid=1861§ionid=146075273. Accessed May 5, 2019.
2. Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Statistics, Prostate Cancer. Bethesda, MD: National Cancer Institute. https://seer.cancer.gov/statfacts/html/prost.html. Accessed May 5, 2019.
3. Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10(2):63-89.
4. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up.Lancet. 2014;384(9959):2027-2035.
5. Ben-Shlomo Y, Evans S, Ibrahim F, et al. The risk of prostate cancer amongst black men in the United Kingdom: the PROCESS cohort study. Eur Urol. 2008;53(1):99-105.
6. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Prostate cancer early detection: version 2.2018. www.nccn.org/professionals/physician_gls/pdf/prostate_detection.pdf. Accessed May 01, 2019.
7. US Preventive Services Task Force. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(18):1901-1913.
8. Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. J Urol. 2013;190(2):419-426.
9. Summary of Safety and Effectiveness Data (SSED). Sangia Total PSA Test – P170037. www.accessdata.fda.gov/cdrh_docs/pdf17/p170037b.pdf. Accessed May 22, 2019 and September 18, 2019.
10. FDA. Medical devices. Sangia Total PSA Test – P170037. www.fda.gov/medical-devices/recently-approved-devices/sangia-total-psa-test-p170037. Accessed May 1, 2019.
11. Sangia Total PSA Test – P170037. www.accessdata.fda.gov/cdrh_docs/pdf17/p170037c.pdf. Accessed April 29, 2019.
12. PMA P170037: FDA Summary of Safety and Effectiveness Data. www.accessdata.fda.gov/cdrh_docs/pdf17/p170037b.pdf. Accessed September 19, 2019.
13. CLIA. Test complexities. CDC. www.cdc.gov/clia/test-complexities.html. Accessed September 18, 2019.
14. 42 CFR § 493.1423 – Standard; Testing personnel qualifications. CFR. US Law. LII/Legal Information Institute. Code of Federal Regulations. www.law.cornell.edu/cfr/text/42/493.1423. Published 1993. Accessed September 18, 2019.
15. Fong T. Opko preps Claros 1 POC platform for quantitative immunodiagnostics market. www.360dx.com/business-news/opko-preps-claros-1-poc-platform-quantitative-immunodiagnostics-market. Accessed September 20, 2019.
16. Risk Factor–42B2. www.sec.gov/Archives/edgar/data/944809/000119312519029612/d699648d424b2.htm. AccessedSeptember 19, 2019.
17. OPKO Health, Inc. www.opko.com/contact. Accessed June 22, 2019.
To comment on this article, contact rdavidson@uspharmacist.com.





