US Pharm. 2012;37(4):36-39.
Nearly everyone has experienced the pain of sunburn at some point. Rates of sunburn are difficult to determine. In a survey of 10,000 teenagers, 83% reported having at least one sunburn in the past summer, and 36% reported having three or more.1 Public awareness of the detriments of sun exposure and the necessity of using sunscreen for skin protection has increased. The large number of available products makes it difficult for consumers to recognize and accurately comprehend what is represented on labels while also understanding what is required for adequate sun protection.
Ultraviolet (UV) Radiation
UV radiation is a type of invisible light emitted by the Sun. The Earth’s surface and its inhabitants are exposed to the entire spectrum of UV radiation, which consists of wavelengths of 290 to 400 nm. For discussion purposes, UV radiation may be separated into two components: UVA and UVB. Wavelengths of 280 to 320 nm constitute UVB radiation, while those of 320 to 400 nm constitute UVA radiation.2 UVB wavelengths generally penetrate epidermal cells, causing DNA and protein damage, and are largely responsible for sunburn. UVA is absorbed by skin cells in the same way as UVB radiation, but it penetrates deeper into the dermis layers.3 It has been shown that UVA radiation exposure results in a wide variety of dermatologic consequences, such as DNA and tissue damage, while also contributing to skin aging.4
Sunburn Protection Factor (SPF)
SPF is the numeric measurement of how effective a sunscreen is at preventing sunburn.2 SPF is calculated by dividing the amount of UV rays required to produce minimal erythema on skin to which a sunscreen product has been applied by the amount of UV rays required to produce minimal erythema on unprotected skin.
Historically, SPF values have ranged from 2 to greater than 100. This has led to an assumption that products with higher SPFs have significantly better sun protection (i.e., SPF 30 being twice as protective as SPF 15). This assumption may be inaccurate because an SPF 15 product blocks about 93% of UVB rays from penetrating the skin, while an SPF 30 product blocks about 97% of UVB rays. Historically, SPF measurements were quantified solely by UVB protection, with possibly no UVA coverage included in the product.5
FDA-Approved Products
With all of the sunscreen options on the market today, there is a wide assortment of active ingredients. It is important to distinguish which active ingredients products contain because some products offer only UVA or UVB coverage. Chemical (organic) absorbers work by absorbing and converting UV radiation into energy before it can cause harm.5 Physical (inorganic) blockers physically block light by reflecting and scattering it over an extensive range of wavelengths.5 Currently, 17 active ingredients are approved by the FDA (TABLE 1).6
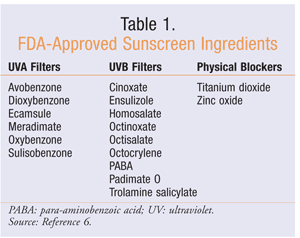
FDA Sunscreen Regulations
To enhance consumer understanding of the growing number of available sunscreen products with ever-expanding SPF claims, the FDA proposed new labeling guidelines for sunscreen manufacturers in July 2011. These new regulations, which will take effect in July 2012, aim to foster better consumer understanding while standardizing requirements for manufacturers. The regulations specify which tests and results are necessary for sun protection claims.6 Key aspects of the regulations, which are discussed below, appear in TABLE 2.6
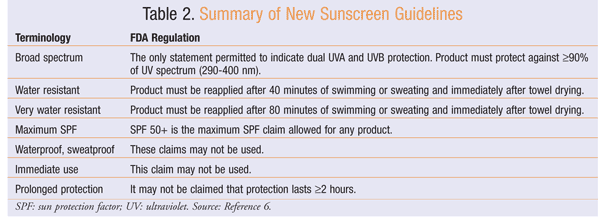
Broad-Spectrum Status: The term broad spectrum has been included on sunscreen product labels in the past; however, there was no universal definition of what this claim represented. A broad-spectrum test is now used by the FDA to assess protection against both UVB and UVA wavelengths. In order to carry a label claim that it is broad spectrum, a product must pass a standard test measuring coverage against at least 90% of the absorbable UV spectrum from 290 to 400 nm. Broad spectrum is the only term that may be used to indicate dual UVB and UVA protection.6
The new guidelines are intended to prevent false labeling claims. Sun protection products that are not broad spectrum or are broad spectrum with an SPF of 2 to 14 must state: “These products have not been shown to protect against skin cancer and early skin aging. They have been shown only to help prevent sunburn.”6 Products that are broad spectrum and have an SPF of 15 or greater may state: “If used as directed with other sun protection measures, this product reduces the risk of skin cancer and early skin aging, as well as helps prevent sunburn.”6
Water Resistance: The guidelines also clarify labeling requirements for water-resistance claims. A sunscreen product must undergo immersion testing to qualify for specific statements regarding water resistance. To be labeled water resistant, a product must retain the labeled SPF during two 20-minute immersion tests with 15 minutes of drying time between immersions. A product that retains the stated SPF during four 20-minute immersions with 15 minutes of drying time between immersions may be labeled very water resistant. The labels of water-resistant products must include directions to reapply sunscreen after 40 minutes of swimming or sweating (water-resistant products) or 80 minutes (very-water-resistant products) and to reapply immediately after towel drying. No product may claim to be waterproof or sweatproof.6
SPF Claims: Secondary to concerns that consumer misunderstanding about products with higher SPF values could lead to extended sun exposure, the new regulations stipulate the maximum labeled SPF to be 50 or greater. The FDA has stated that there is a lack of evidence that products with SPF exceeding 50 provide additional clinical benefit compared with SPF 50 products.6
Additional Comments: The new regulations also emphasize the importance of administering sunscreen products 15 to 30 minutes before initial sun exposure. Other recommendations are to reapply sunscreen at least every 2 hours, with more frequent reapplication following swimming, sweating, or towel drying. Products may not state that they are for “immediate use” or provide protection for longer than 2 hours, unless the manufacturer submits data to support the claim and receives FDA approval.6
Controversies Associated With Sunscreen
Even though the American Academy of Dermatology (AAD) recommends that everyone use broad-spectrum SPF 30 products in addition to other sun-protective strategies, the long-term benefits of sunscreen have not been clearly established.7 There are also concerns about repetitive use and misconceptions about current claims. It is important for pharmacists to understand the following controversies in order to properly advise and educate patients.
Skin Cancer: The two most common types of skin cancer are melanoma and nonmelanoma. Nonmelanoma skin cancers—which are categorized as basal cell carcinoma and squamous cell carcinoma—are the most common type of cancer in the United States, with more than 2 million patients diagnosed in 2010.8 The number of new nonmelanoma cases exceeds the number of all other cancer diagnoses combined, and the incidence rate continues to rise rapidly. Nonmelanoma skin cancers are rarely metastatic and generally have a good prognosis, but they can cause substantial local destruction and disfigurement. There are many identified risk factors, but sun exposure remains the most recognized.8 A randomized, controlled trial evaluating the effect of daily sunscreen use found it promising for preventing the development of squamous cell carcinoma, but additional trials have yielded conflicting results.9,10
Melanoma, another type of skin cancer, has a higher mortality rate, with an estimated 68,000 new cases diagnosed in 2010 and about 8,700 deaths in the U.S. Melanoma ranks second to leukemia in terms of lost years of potential life per death, since the average age at diagnosis is only 59 years. Incidence rates of melanoma have continued to increase dramatically. The lifetime risk of developing melanoma in 1930 was 1/1,500 patients, compared with the lifetime risk of 1/55 in 2005. This dramatic increase may be attributed to sun exposure.11 A meta-analysis of 18 trials assessing the use of sunscreen and rates of melanoma yielded conflicting results.12
Overall, randomized trials evaluating the beneficial effects of sunscreen application in reducing cancer rates inherently contain many confounding variables, including unknown sunscreen SPF, insufficient information about appropriate sunscreen quantity, and time in the sun without reapplication. Although evidence supporting the use of sunscreen to prevent skin cancer is weak, experts agree that public education should focus on appropriate sun protection to reduce rates of skin cancer.
Vitamin D: For the body to synthesize vitamin D, exposure to UVB is necessary, and up to 90% of one’s required vitamin D is formed this way.13 Therefore, there is concern that blocking UVB rays could lead to vitamin D deficiency. While the AAD previously stated that there was no link between vitamin D deficiency and sunscreen use, it recently revised its stance, noting that routine use of sunscreen is associated with an increased likelihood of vitamin D deficiency but that this can be overcome with vitamin D supplementation.13 There has been no clear clinical evidence in controlled settings to prove that this is the case, but even given the possible association, the benefits of sun protection would likely outweigh the risk of vitamin D insufficiency.14
Sensitivity/Toxicity: Sensitivity and toxicity from regular sunscreen use are uncommon and subjective and usually manifest as localized burning and stinging. Contact dermatitis is rarely caused by the ingredients in sunscreen, but the most likely offenders are PABA (para-aminobenzoic acid) and oxybenzone. Organic filters such as avobenzone, sulisobenzone, octinoxate, and padimate O are better tolerated. Individuals at higher risk for sunscreen sensitivity include those with photodermatoses or eczema.13 Although dermatitis is unlikely, if it does occur, a variety of products are available with numerous active ingredient combinations, so most individuals can find a product that is tolerated.5
Hormonal Effects: Hormonal adverse effects—particularly estrogenic effects—related to routine use of sunscreen have been reported.5 Concerns are based on studies of mostly animal models in which breast cancer cells exposed to oxybenzone had a resultant increase in cell proliferation. However, this in vitro evidence does not necessarily correspond with in vivo evidence, as humans may not be exposed to significant quantities of sunscreen. A study of 32 human subjects demonstrated that sunscreen had no effect on hormone levels following application of UV filters daily for 5 days.15 The hormonal effects of sunscreen, particularly long-term safety, remain a controversial issue, as no long-term studies have been conducted and the quantity and routine frequency of sunscreen use may vary significantly between individuals.
SPF and Sun Exposure: It is a common misconception that applying sunscreen, particularly higher-SPF sunscreen, allows one to stay in the sun longer with no increased risk of sun damage. To estimate the maximum sun exposure time before sunburn will develop, multiply the amount of minutes it usually takes a person to burn by the SPF. If it would take 10 minutes for unprotected skin to sunburn, then using a product with a SPF of 15 should prevent skin from getting sunburned for 15 times longer, or about 2.5 hours. However, there are many confounding variables with this calculation.
SPF testing uses a steady amount of UV rays to produce erythema, but the amount of UV rays present varies depending according to the time of day. The midday hours (10 AM-2 PM) typically produce many more UV rays than other times of day, so the length of time one is protected from burning varies throughout the day, with much shorter periods of time during the midday hours. Additional confounding variables include geographic location, altitude, and current weather conditions. Education is important because, while patients may think they are protecting themselves by using sunscreen, they may actually be doing more harm than good if they remain exposed to sunlight for prolonged periods without reapplying sunscreen at least every 2 hours.
Application Thickness: Many consumers believe that simply applying just enough sunscreen to cover exposed skin is sufficient to protect against sun damage. A standard application thickness of 2 g/cm2 is recommended, as this is the thickness used during FDA testing procedures for quantifying the SPF. If a less-than-recommended thickness is used, the actual SPF value of the product may be drastically diminished. A consumer could use an SPF 30 product, but if a lesser thickness is applied, the product may actually be closer to SPF 15. Studies have documented that real-world application practices of sunscreen products typically are closer to 0.5 g/cm2.16 Typically, at least 1 oz. (2 tbsp.) of sunscreen is needed to cover all exposed areas of the arms, legs, neck, ears, and face. For the average-sized person, this equates to the amount it takes to fill a shot glass. An additional amount may be required to cover the back and chest.
Preventive Measures
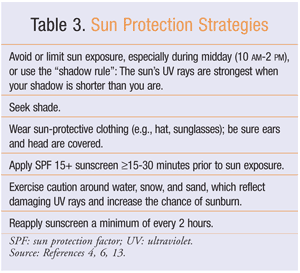
The goal of sunscreen use is to keep people safe in the sun while preventing skin cancers and early aging. Sun protection measures are listed in TABLE 3. Monthly self-examination of common sun-exposed skin areas may help identify early signs of cancer, particularly melanoma. Patients should be educated about the “ABCDEs” (Asymmetry, Border irregularity, Color variation, Diameter >6 mm, Evolution of lesions) of skin cancer so that they can examine their body each month for any new or changing moles. Furthermore, a patient with a family history of melanoma, any previous skin cancers, or a large number of moles should be referred to a dermatologist for a complete skin examination.17
Conclusion
New FDA guidelines aim to enhance consumer understanding of the available sunscreen products while also providing clear regulations for manufacturers regarding determining SPF measurements. Pharmacists must be able to provide education about the importance of sun protection and deliver accurate information concerning patients’ beliefs related to sunscreen.
REFERENCES
1. Geller AC, Oliveria SA, Bishop M, et al. Study of health outcomes
in school children: key challenges and lessons learned from the
Framingham Schools’ Natural History of Nevi Study. J Sch Health. 2007;77:312-318.
2. Nash JF, Tanner PR, Matts PJ. Ultraviolet A radiation: testing and labeling for sunscreen products. Dermatol Clin. 2006;24:63-74.
3. Marrot L, Meunier JR. Skin DNA photodamage and its biological consequences. J Am Acad Dermatol. 2008;58:S139-S148.
4. New (still proposed) rules for sunscreen. UVA testing and ranking may be the most important change. Harv Health Lett. 2010;35:6.
5. Stechschulte SA, Kirsner RS, Federman DG. Sunscreens for non-dermatologists: what you should know when counseling patients. Postgrad Med. 2011;123:160-167.
6. Labeling and effectiveness testing; sunscreen drug products for over-the-counter human use. Fed Regist. 2011;76:35620-35665.
7. American Academy of Dermatology and AAD Association. Position
statement on vitamin D.
www.aad.org/Forms/Policies/Uploads/PS/PS-Vitamin%20D%20Postition%20Statement.pdf.
Accessed December 28, 2011.
8. National Comprehensive Cancer Network. NCCN clinical practice
guidelines in oncology. Basal cell and squamous cell skin cancers. Basal
cell and squamous cell skin cancers. Version 1.2012.
www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Accessed March 7,
2012.
9. Green A, Williams G, Neale R, et al. Daily sunscreen application
and betacarotene supplementation in prevention of basal-cell and
squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet. 1999;354:723-729.
10. van der Pols JC, Williams GM, Pandeya N, et al. Prolonged
prevention of squamous cell carcinoma of the skin by regular sunscreen
use. Cancer Epidemiol Biomarkers Prev. 2006;15:2546-2548.
11. National Comprehensive Cancer Network. NCCN clinical practice
guidelines in oncology. Melanoma. Version 3.2012.
www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf. Accessed
March 7, 2012.
12. Dennis LK, Beane Freeman LE, VanBeek MJ. Sunscreen use and the risk for melanoma: a quantitative review. Ann Intern Med. 2003;139:966-978.
13. Sambandan DR, Ratner D. Sunscreens: an overview and update. J Am Acad Dermatol. 2011;64:748-758.
14. Gilchrest BA. The a-B-C-ds of sensible sun protection. Skin Therapy Lett. 2008;13:1-5.
15. Janjua NR, Mogensen B, Andersson AM, et al. Systemic absorption
of the sunscreens benzophenone-3, octyl-methoxycinnamate, and
3-(4-methyl-benzylidene) camphor after whole-body topical application
and reproductive hormone levels in humans. J Invest Dermatol. 2004;123:57-61.
16. Autier P, Boniol M, Severi G, et al. Quantity of sunscreen used by European students. Br J Dermatol. 2001;144:288-291.
17. Cummins DL, Cummins JM, Pantle H, et al. Cutaneous malignant melanoma. Mayo Clin Proc. 2006;81:500-507.
To comment on this article, contact rdavidson@uspharmacist.com.





