US Pharm. 2023;48(12):HS2-HS10.
ABSTRACT: Stress ulceration poses a significant threat to critically ill patients, necessitating stress ulcer prophylaxis (SUP). While the choice of the optimal prophylactic agent remains uncertain, proton pump inhibitors (PPIs) often take precedence due to their effectiveness in gastric acid suppression. Concerns surrounding PPIs’ potential adverse effects are countered by evidence suggesting that the benefits of SUP outweigh the risks. Recent data even suggest a potential reduction in stress ulcers in ICU patients receiving enteral nutrition. Pharmacists play an indispensable role in SUP, ensuring the safe and effective use of medications, collaborating with healthcare teams, monitoring patients, and educating them on adherence.
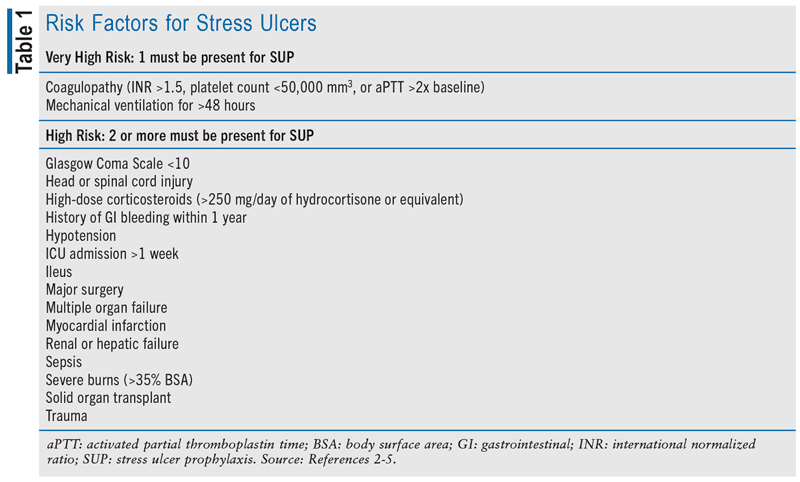
Stress ulceration is the development of superficial ulcers in the upper gastrointestinal (GI) tract (i.e., esophagus, stomach, duodenum) during hospitalization, particularly in the ICU setting.1,2 Stress ulcers are distinctly separate from reactivation of chronic duodenal or gastric ulcers. Stress ulcers develop because of either hypersecretion of acid or impaired mucosal protection secondary to GI-tract hypoperfusion, mucosal ischemia, or disruption.1,2 As such, stress ulcers are typically suspected in patients experiencing hematemesis, melena, anemia, hypotension, or shock. Multiple risk factors have been linked to the risk of stress ulceration (see TABLE 1). However, the two most frequently reported risk factors for stress ulcer development and clinically significant GI bleeding are prolonged mechanical ventilation beyond 48 hours and the presence of coagulopathy.3-5
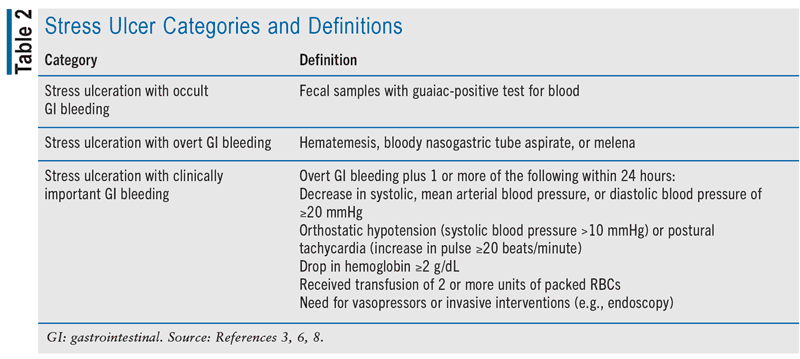
Stress ulcers in critically ill patients can be divided into three categories, each with separate definitions (see TABLE 2).2,3,6,7 Occurrence rates vary based on the classification of stress ulceration, presence of risk factors, and the prophylaxis prescribed. Estimates suggest that asymptomatic stress ulceration is quite common in critically ill patients who do not receive prophylaxis, likely exceeding 75%.8,9 The incidence is lower in those with stress ulceration accompanied by occult bleeding, ranging from 15% to 50%, while overt bleeding due to stress ulceration occurs at a rate of 1.5% to 8.5%.8,9 Stress ulceration leading to clinically significant bleeding affects approximately 1% to 3% of patients in the ICU.8,9
Stress ulceration can lead to serious complications, including perforation, hemorrhagic shock, and death.8,9 Evidence has also suggested an association between stress ulceration and an increased length of stay in the ICU.8 As such, stress ulcer management primarily focuses on preventive measures, commonly referred to as stress ulcer prophylaxis (SUP). These proactive strategies encompass the regular monitoring of hemoglobin levels and screening for occult blood in both feces and gastric contents. Moreover, SUP entails the optimization of prophylactic medications designed to curb the excessive production of gastric acid. While SUP might be considered harmless at first glance, it may lead to unforeseen consequences, as there have been reported associations with an elevated risk of pneumonia and Clostridioides difficile infection.10,11 These reports have triggered discussions and debates regarding the safety of prophylactic strategies. Pharmacists can recommend appropriate therapeutic regimens and strategies for maintaining optimal patient care, avoiding therapeutic risks, and positively impacting health in those with stress ulcers.12
Prophylactic Therapy
The therapeutic landscape of SUP has evolved significantly over the years, guided by a better understanding of risk factors, improved diagnostic tools, and a growing body of evidence. The previous paradigm of indiscriminate prophylactic therapy for all ICU patients has been gradually replaced by a more targeted and individualized approach. This shift has been driven by the desire to minimize potential risks and side effects associated with prophylactic agents while maximizing the benefits for patients who would truly benefit and are considered high- or very-high-risk (see TABLE 1).
The optimal prophylactic choice for an SUP agent remains uncertain, and considerable variation exists in clinical practice when it comes to selecting an agent for critically ill patients. Prophylactic agents for stress ulcers include proton pump inhibitors (PPIs), histamine-2 receptor antagonists (H2RAs), and sucralfate (see TABLE 3). Evidence suggests that PPIs are favored over alternative prophylactic agents such as H2RAs, sucralfate, or antacids.7 In a Cochrane meta-analysis of 18 studies involving 1,636 participants, PPIs were found to be more effective in suppressing gastric acid compared with H2RAs (risk ratio [RR] 2.90, 95% CI 1.83-4.58; absolute risk 4.8%, 95% CI 2.1-9.0).13 Furthermore, a 2020 randomized, controlled trial conducted on critically ill patients demonstrated a statistically significant reduction in GI bleeding among patients using PPIs compared with those using H2RAs (1.3% vs. 1.8%, P = .009).14 However, if patients cannot tolerate a PPI, H2RA is considered the alternative.15,16 In uncommon situations wherein the use of PPIs and H2RAs is not feasible (e.g., drug intolerance or interactions), sucralfate may be considered as an alternative prophylactic option.17 Rarely or if ever, antacids or prostanoids are used.
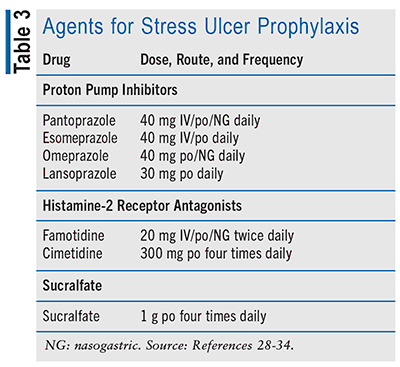
Comparative data indicating the superiority of one PPI over another are lacking. Likewise, when considering H2RAs, there is also a scarcity of data comparing the different options within this class. As such, selection of a prophylactic agent from a particular class is often influenced by hospital formularies, practice protocols, patient characteristics (e.g., comorbidities, drug tolerance), avoidance of potential drug interactions, and cost rather than clinical efficacy between individual agents.
While PPIs might be the favored prophylactic class, concerns have arisen in the past regarding their potential association with the risk of pneumonia, C difficile infection, and mortality.17,18 The development of these risks is related to the increase in gastric pH, thereby leading to bacterial overgrowth. Yet, more recent studies contradict these concerns.13,14,19-23 Therefore, it seems that the advantages of offering SUP surpass the potential risks associated with withholding therapy.
Emerging data suggest that patients in the ICU who are receiving enteral nutrition (EN) may have a lower occurrence of stress ulcers.24-25 A 2018 meta-analysis found that there was no additional benefit of prophylaxis in reducing GI bleeding in patients receiving EN.22 Additionally, the analysis revealed that prophylaxis had no effect on overall mortality, duration of positive pressure ventilation, incidence of C difficile infection, or ICU length of stay.22 However, because data from robust studies are lacking, EN should not replace the use of acid-suppressive therapy.23
Although there is a consensus among most experts that SUP should be used when risk factors are present, there is limited consensus regarding the appropriate discontinuation point.7 There is greater consensus on discontinuing SUP in patients upon their discharge from the ICU, given the minimal risk of stress ulcer bleeding in non-ICU hospitalized patients.26
The Pharmacist’s Role
Pharmacists play a vital role in SUP by ensuring the safe and effective use of medications in preventing stress-related mucosal damage. They assess patients’ risk factors and collaborate with healthcare teams to identify patients in need of SUP. Collaborating closely with other healthcare professionals, pharmacists contribute to evidence-based decision-making and ensure appropriate prophylactic agent selection, drug dosing, and administration. Pharmacists can continuously monitor patients for adverse effects, drug interactions, and therapeutic outcomes, making necessary adjustments to therapy. They also can educate patients and their families about SUP, emphasizing proper medication administration and the importance of adherence. Lastly, pharmacists can also help identify patients for whom SUP is no longer necessary.27
Pharmacists’ expertise in medication management, monitoring, and patient education is integral to minimizing the risk of stress ulcers and their complications, ultimately improving patient outcomes.
Conclusion
Stress ulceration is a significant concern in critically ill patients, with varying levels of risk and complications associated with different categories of stress ulcers. The management of stress ulcers primarily revolves around preventive measures. While the optimal choice of prophylactic agent remains uncertain, PPIs are often favored over alternatives due to their efficacy in suppressing gastric acid. Despite concerns about potential adverse effects associated with PPIs, evidence suggests that the benefits of SUP outweigh the risks. Additionally, recent data hint at a potential reduction in stress ulcers in ICU patients receiving EN. Pharmacists play a crucial role in SUP by ensuring the safe and effective use of medications, collaborating with healthcare teams, monitoring patients, and educating them about the importance of adherence. In a shifting landscape of SUP, pharmacists remain vital in optimizing patient care.
REFERENCES
1. Moody FG, Cheung LY. Stress ulcers: their pathogenesis, diagnosis, and treatment. Surg Clin North Am. 1976;56(6):1469-1478.
2. Quenot JP, Thiery N, Barbar S. When should stress ulcer prophylaxis be used in the ICU? Curr Opin Crit Care. 2009;15(2):139-143.
3. Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. N Engl J Med. 1994;330:377-381.
4. Spirt MJ. Stress-related mucosal disease: risk factors and prophylactic therapy. Clin Ther. 2004;26(2):197-213.
5. ASHP therapeutic guidelines on stress ulcer prophylaxis. ASHP commission on therapeutics and approved by the ASHP board of directors on November 14, 1998. Am J Health Syst Pharm. 1999;56(4):347-379.
6. Cook D, Guyatt G. Prophylaxis against upper gastrointestinal bleeding in hospitalized patients. N Engl J Med. 2018;378(26):2506-2516.
7. Barletta J, Bruno J, Buckley M, Cook D. Stress ulcer prophylaxis. Critic Care Med. 2016;44(7):1395-1405.
8. Cook DJ, Griffith LE, Walter SD, et al. The attributable mortality and length of intensive care unit stay of clinically important gastrointestinal bleeding in critically ill patients. Crit Care. 2001;5(6):368-375.
9. Tsiotos GG, Mullany CJ, Zietlow S, van Heerden JA. Abdominal complications following cardiac surgery. Am J Surg. 1994;167(6):553-557.
10. Miano TA, Reichert MG, Houle TT, et al. Nosocomial pneumonia risk and stress ulcer prophylaxis: a comparison of pantoprazole vs ranitidine in cardiothoracic surgery patients. Chest. 2009;136(2):440-447.
11. Ro Y, Eun CS, Kim HS, et al. Risk of Clostridium difficile infection with the use of a proton pump inhibitor for stress ulcer prophylaxis in critically ill patients. Gut Liver. 2016;10(4):581-586.
12. Mousavi M, Dashti-Khavidaki S, Khalili H, et al. Impact of clinical pharmacy services on stress ulcer prophylaxis prescribing and related cost in patients with renal insufficiency. Int J Pharm Pract. 2013;21(4):263-269.
13. Toews I, George AT, Peter JV, et al. Interventions for preventing upper gastrointestinal bleeding in people admitted to intensive care units. Cochrane Database Syst Rev. 2018;6(6):CD008687.
14. Young PJ, Bagshaw SM, Forbes AB, et al. Effect of stress ulcer prophylaxis with proton pump inhibitors vs histamine-2 receptor blockers on in-hospital mortality among ICU patients receiving invasive mechanical ventilation: the PEPTIC randomized clinical trial. JAMA. 2020;323(7):616-626.
15. Wang Y, Ye Z, Ge L, et al. Efficacy and safety of gastrointestinal bleeding prophylaxis in critically ill patients: systematic review and network meta-analysis. BMJ. 2020;368:l6744.
16. Mohebbi L, Hesch K. Stress ulcer prophylaxis in the intensive care unit. Proc (Bayl Univ Med Cent). 2009;22(4):373-376.
17. MacLaren R, Reynolds PM, Allen RR. Histamine-2 receptor antagonists vs proton pump inhibitors on gastrointestinal tract hemorrhage and infectious complications in the intensive care unit. JAMA Intern Med. 2014;174(4):564-574.
18. Alhazzani W, Alshamsi F, Belley-Cote E, et al. Efficacy and safety of stress ulcer prophylaxis in critically ill patients: a network meta-analysis of randomized trials. Intensive Care Med. 2018;44(1):1-11.
19. Krag M, Marker S, Perner A, et al. Pantoprazole in patients at risk for gastrointestinal bleeding in the ICU. N Engl J Med. 2018;379(23):2199-2208.
20. Alhazzani W, Alenezi F, Jaeschke RZ, et al. Proton pump inhibitors versus histamine-2 receptor antagonists for stress ulcer prophylaxis in critically ill patients: a systematic review and meta-analysis. Crit Care Med. 2013;41(3):693-705.
21. Lin PC, Chang CH, Hsu PI, et al. The efficacy and safety of proton pump inhibitors vs histamine-2 receptor antagonists for stress ulcer bleeding prophylaxis among critical care patients: a meta-analysis. Crit Care Med. 2010;38(4):1197-1205.
22. Huang HB, Jiang W, Wang CY, et al. Stress ulcer prophylaxis in intensive care unit patients receiving enteral nutrition: a systematic review and meta-analysis. Crit Care. 2018;22(1):20.
23. Barletta JF. Prophylactic acid suppression and enteral nutrition. Curr Opin Clin Nutr Metab Care. 2023;26(2):174-178.
24. Ohbe H, Morita K, Matsui H, et al. Stress ulcer prophylaxis plus enteral nutrition versus enteral nutrition alone in critically ill patients at risk for gastrointestinal bleeding: a propensity-matched analysis. Intensive Care Med. 2020;46(10):1948-1949.
25. Bonten MJ, Gaillard CA, van Tiel FH, et al. Continuous enteral feeding counteracts preventive measures for gastric colonization in intensive care unit patients. Crit Care Med. 1994;22(6):939-944.
26. Herzig SJ, Vaughn BP, Howell MD, et al. Acid-suppressive medication use and the risk for nosocomial gastrointestinal tract bleeding. Arch Intern Med. 2011;171(11):991-997.
27. Cascone AE, Sullivan J, Ackerbauer K, et al. Pharmacist-initiated de-prescribing efforts reduce inappropriate continuation of acid-suppression therapy initiated in the ICU. Am J Med. 2023;136(2):186-192.
28. Protonix (pantoprazole) package insert. Konstanz, Germany: Pfizer; May 2012.
29. Nexium (esomeprazole) package insert. Wilmington, DE: AstraZeneca Pharmaceuticals, LP; December 2014.
30. Prilosec (omeprazole) package insert. Wilmington, DE: AstraZeneca Pharmaceuticals, LP; September 2012.
31. Prevacid (lansoprazole) package insert. Deerfield, IL: Takeda Pharmaceuticals America, Inc.; September 2012.
32. Pepcid (famotidine) package insert. Bridgewater, NJ: Valeant Pharmaceuticals North America, LLC; June 2018.
33. Cimetidine package insert. Etobicoke, Ontario: Mylan Pharmaceuticals, ULC; September 2009.
34. Carafate (sucralfate) package insert. Bridgewater, NJ: Aptalis Pharma US, Inc.; March 2013.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






